Unlocking the Role of Long Non-Coding RNAs in Multiple Myeloma: The LINC01432–CELF2 Axis
Published in Cancer, Protocols & Methods, and Cell & Molecular Biology
Introduction
Multiple myeloma (MM) remains a challenging blood cancer with frequent relapse despite major therapeutic advances. My lab is dedicated not only to uncovering the molecular mechanisms that drive this disease but also to training the next generation of scientists who will shape the future of biomedical research. This study perfectly reflects that mission, featuring contributions from high school, undergraduate, and graduate students, a postdoctoral fellow, and bioinformatics trainees, supported by experts across RNA biology, cancer biology, immunology, and genetics.
The Story Behind the Discovery
This project began when I joined Washington University School of Medicine and started exploring the molecular mechanisms underlying multiple myeloma in collaboration with renowned clinicians and fellow genomicists. Together, we used next-generation sequencing to analyze samples from newly diagnosed multiple myeloma patients, aiming to understand how long non-coding RNAs (lncRNAs) contribute to disease progression and relapse-free survival following standard therapies.
Our analyses identified LINC01432 as one of the most significantly dysregulated lncRNAs associated with patient outcomes. Through biochemical and functional studies, we discovered that LINC01432 binds to the RNA-binding protein CELF2, forming a complex that inhibits apoptosis and modulates immune response pathways. Functionally, LINC01432 depletion increased apoptosis, while its overexpression promoted myeloma cell survival.
Looking Forward
This LINC01432-CELF2 axis reveals a previously unrecognized mechanism of myeloma persistence. By integrating clinical genomics with mechanistic RNA biology, our work demonstrates how lncRNA-protein interactions shape cancer cell behavior and influence therapeutic response.
Beyond the science itself, this study showcases the impact of collaborative, trainee-driven research, illustrating how mentorship, curiosity, and teamwork can lead to discoveries that both advance cancer biology and inspire the next generation of biomedical researchers.
Read the full paper:
Mishra R, Thunuguntla P, Duraiyan D, Perkin A, Bagwill K, Gonzales S, Sizemore C, Brizuela V, King J, Daly S, Chang YJ, Abebe M, Rajana Y, Wichmann K, Enyan C, Rangineni S, Fiala M, Fortier J, Jayasinghe R, Schroeder M, Ding L, Vij R, DiPersio J, Silva-Fisher J.
Investigation of lncRNA expression in newly diagnosed multiple myeloma reveals a LINC01432–CELF2 axis as an inhibitor of apoptosis.
Oncogenesis. 2025 Oct 6;14(1):36. doi: 10.1038/s41389-025-00579-w. PMID: 41052980; PMCID: PMC12500896.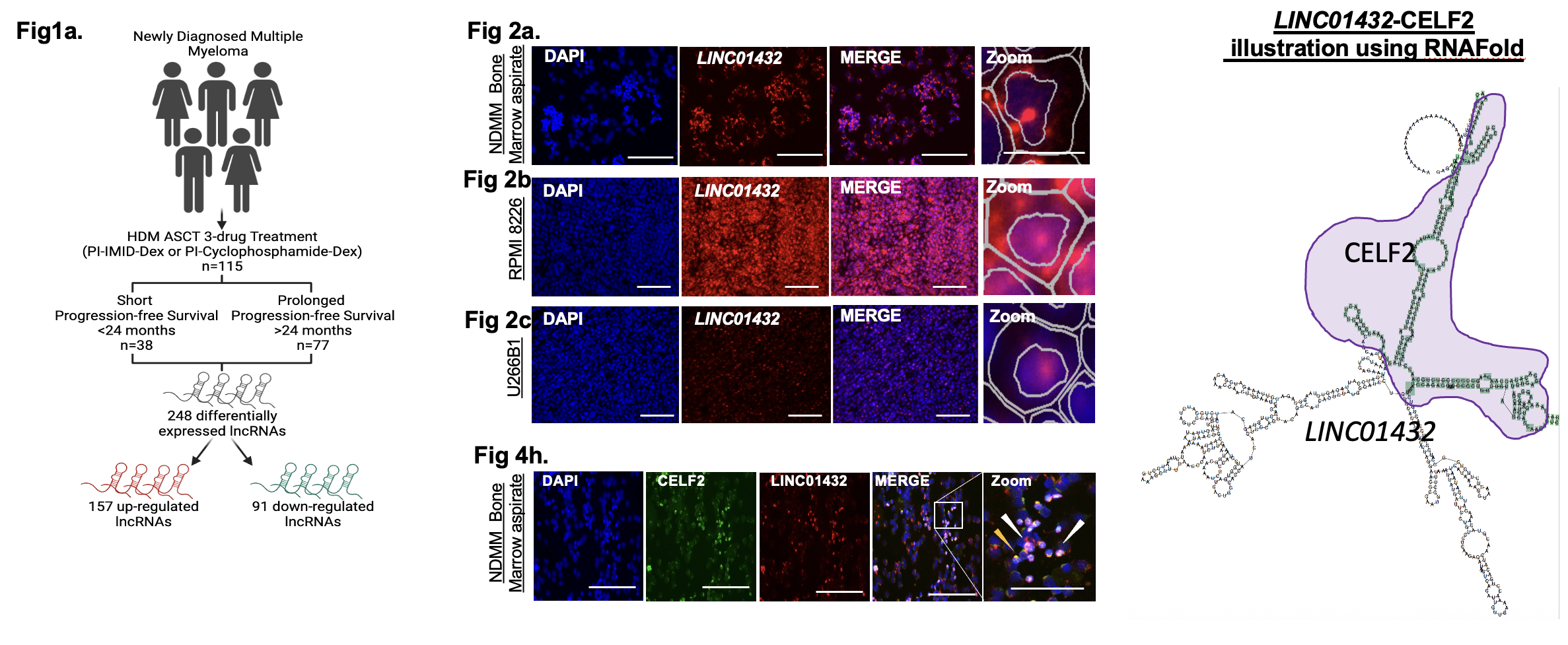
Follow the Topic
-
Oncogenesis

A peer-reviewed open access online journal that publishes articles exploring mechanistic insight and molecular basis of cancer and related phenomena. It seeks to promote diverse and integrated areas of molecular biology, cell biology, oncology, and genetics.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
A few highlights from the paper:
• Key finding: The LINC01432–CELF2 axis (Long non-coding RNA-protein interaction) suppresses apoptosis in multiple myeloma, highlighting a novel mechanism of disease progression.
• Approach: Combined RNA-seq profiling, ChIRP/iCLIP pulldown assays, and CRISPR-mediated functional assays identified LINC01432 as a top lncRNA associated with poor prognosis. CRISPR-mediated knockdown of LINC01432 expression results in upregulation of genes associated with interferon-α/γ responses and increases apoptosis.
• Significance & future steps: Targeting the LINC01432–CELF2 interaction may offer a new therapeutic dimension for treatment-resistant multiple myeloma.