Using population-based surveys to capture more complete estimates of community infection levels in NYC during Omicron BA.2/BA.2.12.1 surge
Published in Healthcare & Nursing, Biomedical Research, and Public Health
The high transmissibility of the SARS-CoV-2 Omicron variant that emerged in late 2021 led to a rapid rise in community spread of infections1. A population with substantial levels of immunity, either due to vaccines and/or prior infections, complicates the interpretation of transmission trends and severity2. At that point in time, there were important epidemiologic questions about the variant – such as how widely it was spreading, who were the key sub-populations that were getting infected, how severe it was, and whether high levels of immunity from infections acquired during the recent BA.1 surge, as well as whether hybrid immunity helped to moderate SARS-CoV-2 transmission in the population.
These questions, which arise during any major surge, have become increasingly challenging to answer for several reasons. First, case and testing-based metrics reflect only individuals who present to care, and these individuals may differ meaningfully from the general population on socio-economic and demographic characteristics, as well as infection risk factors. Importantly, we also could not rule out incomplete reporting of cases by health providers, particularly during surges when high transmission and testing demand can hamper complete and timely case reporting. Additionally, at that time there was an increasing proportion of people with COVID who tested exclusively with at-home rapid tests, which are not reported to the department of health and not reflected in case counts3. Finally, the uptake of COVID vaccines and hybrid immunity may have altered the clinical presentation (i.e., severity) of infection2, which could have affected decisions around test seeking, especially if symptoms among those with vaccine- and/or infection-induced immunity are less severe.
Because of these concerns and challenges, we fielded a population-representative survey designed to get around some of these issues. The survey randomly sampled adults in New York City (NYC) during the Omicron BA.2/2.12.1 surge during a 2-week period in April 2022. Our survey collected a small set of pertinent indicators on recent SARS-CoV-2 diagnostic testing encounters, test results, reported symptoms and close contact with confirmed cases, as well as prior infection and vaccination status. This approach, for example, could help quantify the increased uptake of at-home testing, including among those with positive test results. We also wanted to triangulate our survey findings with data on hospitalizations, deaths, and concentration of SARS-CoV-2 virus in wastewater to gain insights around the extent and severity of transmission in the community.
Our survey estimated that 22.1% of NYC adults had an active SARS-CoV-2 infection during the two-week survey period, which was equivalent to ~1.5 million adults. The estimate was 29-fold higher than the 51,218 detected by routine case surveillance based on testing. Our more complete estimate included roughly 7% that were positive exclusively based on an at-home rapid antigen test that was not followed by a confirmatory diagnostic test (i.e., point-of-care rapid antigen or PCR test), as well as about 4% who were possible infections based on reported symptoms and having been in close contact with a confirmed case but did not test at all. Further, we found that the prevalence of SARS-CoV-2 infection was higher among adults with co-morbidities, among those 65+ years, and among unvaccinated adults. Among adults with a SARS-CoV-2 infection, 66.2% had hybrid immunity (due to both vaccination and prior infection) compared to 46.3% among those without an infection.
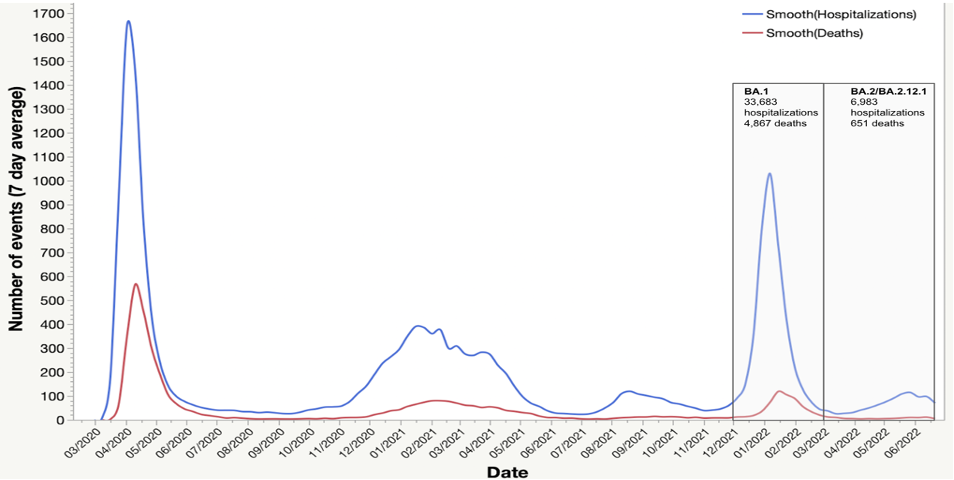
To better ascertain the severity of the BA.2/BA.2.12.1 sub-variant, we examined routinely collected data on hospitalizations and deaths. We found that while COVID-19 hospitalizations and deaths increased during the BA.2/BA.2.12.1 surge, the increase was modest when compared to the BA.1 surge (Figure 1). Our survey findings of the high prevalence of hybrid immunity among those who were infected likely explain the lower rates of hospitalizations and deaths in NYC during this surge.
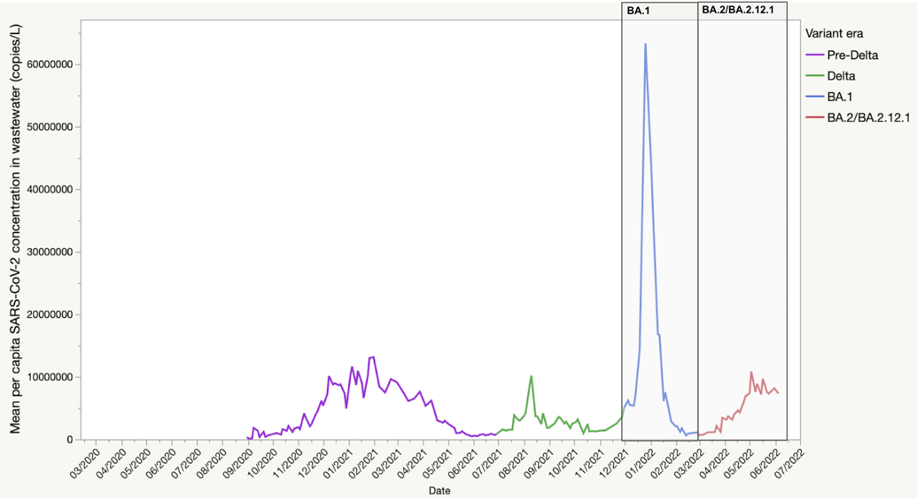
We also examined the signal of SARS-CoV-2 concentration per capita in NYC wastewater surveillance data during the BA.2 surge which was modest in comparison to that during the BA.1. surge (Figure 2). This finding prompted us to hypothesize whether this modest peak could reflect lower mean peak viral load per infection due to greater acquired T-cell mediated immunity from recent prior BA.1 infection4, which infected large swaths of NYC adults in late 2021 early 20225. If this were true, re-infections may reduce the magnitude of peak SARS-CoV-2 concentration in wastewater, even for roughly the same number of individuals in the population with active SARS-CoV-2 infection.
Our study showed that strategically and rapidly deployed population-representative surveys can answer important questions about the extent and impact of SARS-CoV-2 pandemic surges. Our findings showed that traditional case-based surveillance vastly under-estimated case burden and tends to be missing information on the key sub-populations with the highest burden of infections (e.g., older, and unvaccinated adults). Timely and accurate data on transmission by sub-groups is critical for informing programmatic interventions for addressing disparities in testing and vaccination uptake.
Our findings also addressed questions about the profile of people with active SARS-CoV-2 infections during the BA.2 surge. Our findings highlighted that collecting information on population-level immunity due to vaccines and/or prior infections may help epidemiologists interpret the severity of new surges. Furthermore, by contextualizing population-based surveys with wastewater concentration of SARS-CoV-2 virus, our findings pointed to current knowledge gaps that exist related to the utility of wastewater surveillance to make inferences about the magnitude of community transmission – especially against a dynamic landscape of population-level immunity6. Population-based surveys, especially if routinely or strategically deployed, can strengthen the utility of wastewater surveillance data.
Our study demonstrates that randomly sampling respondents through population-based surveys can be a powerful tool that allows us to make inferences about SARS-CoV-2 transmission in the community7. We believe our approach can supplement traditional public health surveillance approaches to make sense of transmission patterns - both for COVID-19 but also for other epidemic infections of public health significance8,9. For more on the use of survey-based approaches to assess the true burden of infection during SARS-CoV-2 surges, see our recently published commentary9.
- Iuliano AD, Danielle Iuliano A, Brunkard JM, et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods — United States, December 2020–January 2022. MMWR Morbidity and Mortality Weekly Report. 2022;71(4):146-152. doi:10.15585/mmwr.mm7104e4
- Bhattacharyya RP, Hanage WP. Challenges in inferring intrinsic severity of the SARS-CoV-2 omicron variant. N Engl J Med. 2022;386(7):e14.
- Rader B, Gertz A, Iuliano AD, et al. Use of At-Home COVID-19 Tests - United States, August 23, 2021-March 12, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):489-494.
- Puhach O, Adea K, Hulo N, et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat Med. 2022;28(7):1491-1500.
- Qasmieh SA, Robertson MM, Teasdale CA, Kulkarni SG, Nash D. Estimating the period prevalence of SARS-CoV-2 infection during the Omicron (BA.1) surge in New York City (NYC), January 1-March 16, 2022. Clin Infect Dis. Published online August 12, 2022. doi:10.1093/cid/ciac644
- Armas F, Chandra F, Lee WL, et al. Contextualizing Wastewater-Based surveillance in the COVID-19 vaccination era. Environ Int. 2023;171(107718):107718.
- Dean N. Tracking COVID-19 infections: time for change. Nature. 2022;602(7896):185.
- Jernigan DB, George D, Lipsitch M. Learning from COVID-19 to improve surveillance for emerging threats. Am J Public Health. 2023;113(5):520-522.
- Qasmieh, S., Robertson, M. & Nash, D. “Boosting” surveillance for a more impactful public health response during protracted and evolving infection disease threats: insights from the COVID-19 pandemic. Health Security (in press). Health Secu. (in press)
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in