Virulence factors not required?
Published in Microbiology
Microbial pathogens cause devastating infections that threaten our wellbeing, and in some cases, even our survival. According to Koch’s postulates, these pathogens can be isolated from active infections. Once isolated, experimental models can help us get to know our microbial adversaries and how they are so successful in invading and damaging our tissues. To study this impressive arsenal of microbial pathogenicity strategies, we can use a wide range of infection models spanning from infections in laboratory animals (in vivo) to infections of single cell tissues in a culture dish (ex vivo or in vitro). But do these pathogens always show their true face when we examine them in the lab?
Imagine you isolated the opportunistic pathogenic yeast Candida albicans from a patient experiencing devastating chronic infections. As you read up on this organism, you will learn that it is usually an otherwise harmless commensal member of our normal microbiota, which only causes infections when the conditions are right. But don’t be fooled, this otherwise “harmless” microbe boasts a repertoire of virulence mechanisms including adhesins, invasins, proteases, a cytolytic toxin, and can grow in filamentous forms that allow deep tissue invasion. Surprisingly, when infecting epithelial tissues with the clinical isolate ex vivo, no hostile pathogenic activities can be detected. You do not understand why this seemingly harmless pathogen causes so devastating infections in the patient it came from. Exactly this paradox sparked the central question of our study.
As a commensal that has efficiently co-evolved with us humans as its host, C. albicans is remarkably adapt at sensing and responding to changes in its host environment. Even anticipatory responses to prepare for attack by activated immune defences are not unheard of. While our lab was investigating how C. albicans can sense and anticipate immune mediators, we received feedback suggesting that we should include an inert protein like human serum albumin as a control. In doing so, we found that albumin was anything but inert, and rather exhibits a tremendous potential to boost fungal growth. Intrigued by this, we systematically explored the role of albumin in C. albicans epithelial infections by supplementing it in well-established in vitro infection models.
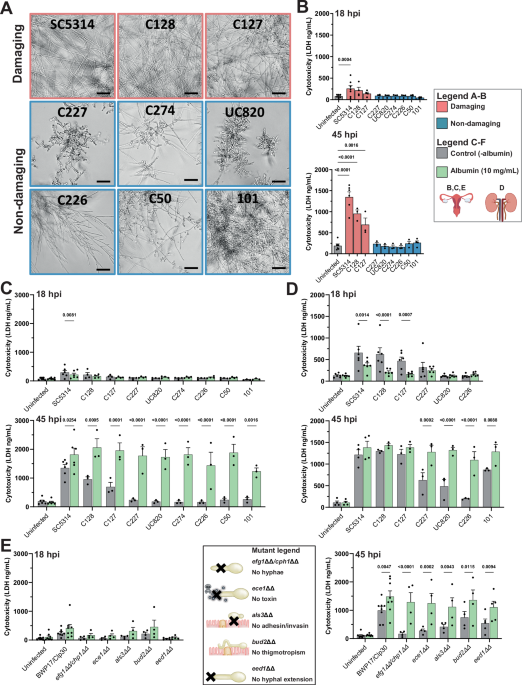
When performing infections with a range of clinical C. albicans isolates, we found that the presence of albumin enhanced the ability of otherwise avirulent C. albicans strains to cause tissue damage. As we found that even deletion mutants in well-established crucial virulence factors could suddenly exhibit pathogenicity again, we knew that we had to go off the beaten path to unveil a hidden pathogenic potential that standard models failed to capture so far.
Intrigued by the growth-promoting potential of albumin, we studied changes in the fungal metabolism that can accommodate this tremendous increase in growth. One might think that this increased growth was simply the consequence of providing the fungus with extra amino acids, but there was way more behind it. We found that in response to albumin C. albicans undergoes a full metabolic reprogramming. Albumin fuelled amino acid metabolism, increased activity of the tricarboxylic acid cycle, and mitochondrial respiration. In parallel, the glutathione pathway, an important oxidative stress detoxification pathway, was also strongly increased.
Through characterising alterations in gene expression, we were able to observe transcriptionally regulated adaptations leading to increased biofilm formation, as well as the fungal transcription factor STP2 and the protein kinase IRE1 as crucial players regulating amino-acid metabolism and iron metabolic processes respectively. These two genes were crucially linked with the albumin-induced pathogenicity.
With the increased metabolic turnover, the fungus also released a variety of metabolites into its environment, particularly unsaturated fatty acids. One of these, the oxidised lipid 13-hydroxyoctadecadienoic acid (13-HODE), was linked with the ability of albumin to enhance the damage caused by C. albicans. Altogether, albumin seemed to drive increased biofilm formation that releases cytotoxic metabolites in close vicinity of the host epithelium.
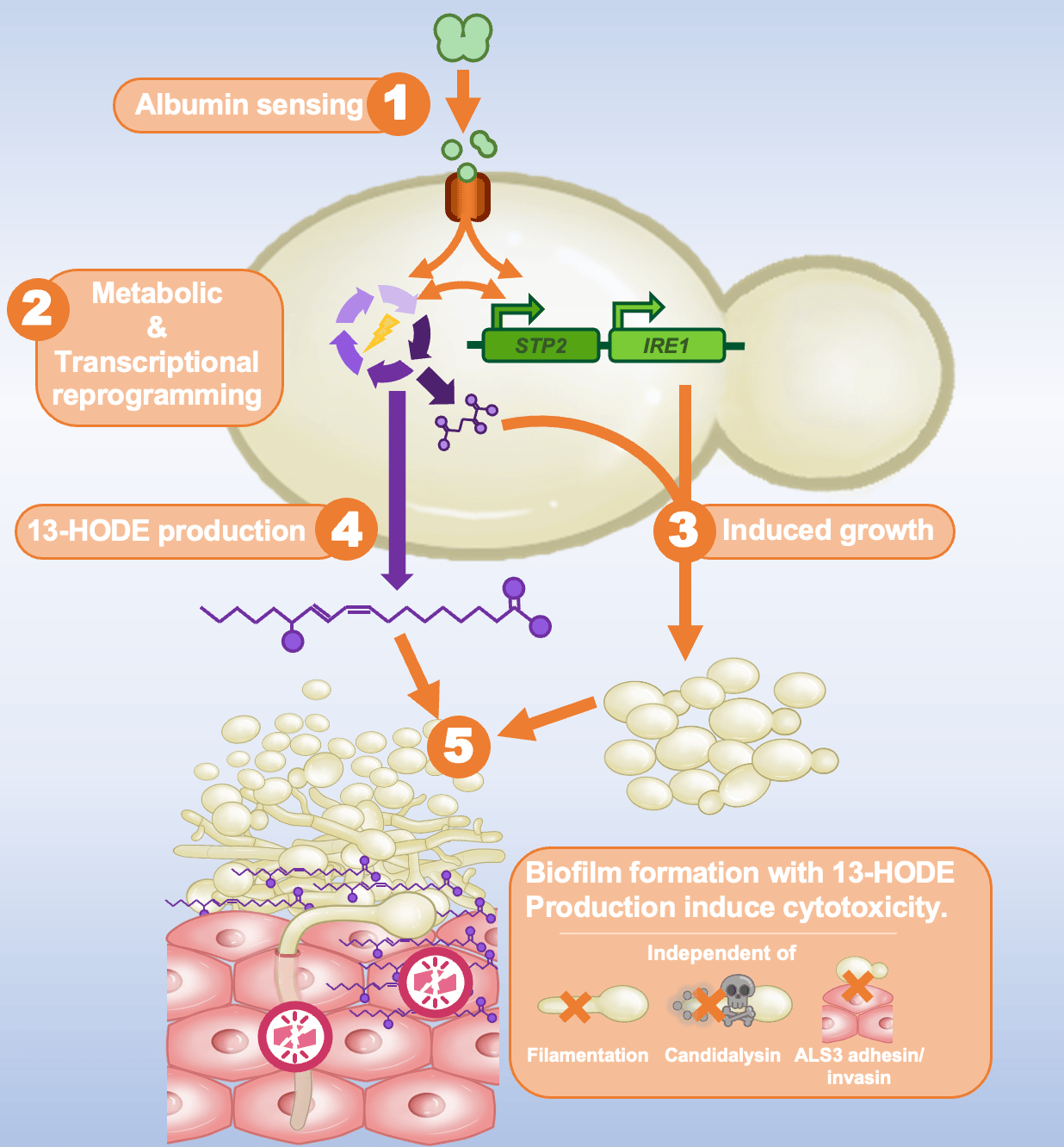
Candida albicans undergoes metabolic and transcriptional reprogramming in response to human serum albumin, leading to biofilm formation and release of oxidised lipids that can cause cytotoxicity to host epithelial tissues
In conclusion, our study not only revealed an alternative virulence mechanism of C. albicans, but also provided an explanation for the discrepancy between the in vivo virulence potential of clinical C. albicans isolates and their ability to cause tissue damage ex vivo in experimental models. Furthermore, the study highlights the importance of considering relevant host factors when investigating host-pathogen interactions in vitro.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in