Visible-Light Photocatalytic Di- and Hydro-Carboxylation of Unactivated Alkenes with CO2
Published in Chemistry

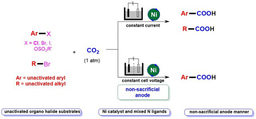
Carbon dioxide (CO2) is a greenhouse gas that has an important impact on climate change, while it is also considered to be an abundant, cheap, readily available, non-toxic and renewable one-carbon (C1) building block.1 It is of great significance to use CO2 to participate in chemical transformation and accurate synthesis of high value-added chemicals. Particularly, it is an important direction in synthetic chemistry to transform CO2 to valuable carboxylic acids and derivatives, which have important applications in medicine and polymer industry. Although promising progress has been made, it still meets many limitations.2 On the other hand, photocatalysis has witnessed dramatic developments over the past decades due to its environmental friendliness, mild conditions and high functional group compatibility.3-4 Considering the extensive source of alkenes, chemists have realized a series of alkene carboxylation reactions featuring different chemo- and regio-selectivities with CO2 via visible-light photocatalysis, affording various types of carboxylic acids and derivatives.5-8 However, most of the examples are limited to activated alkenes (e.g., aryl alkenes or acrylates).9-10 In contrast, the unactivated alkenes,11-12 which are more common in nature and industry but are more challenging substrates as well, have been rarely studied in visible-light photocatalytic carboxylation with CO2 due to their low reactivity. Therefore, it is of great significance to develop a new strategy to realize the visible-light-induced carboxylation of unactivated alkenes with CO2. 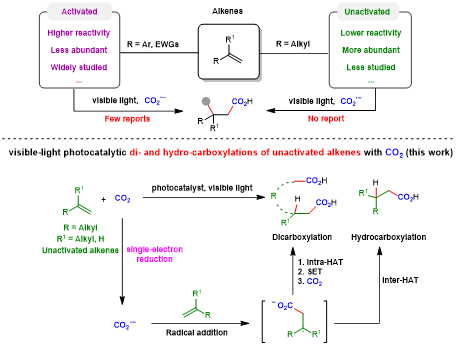
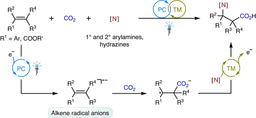
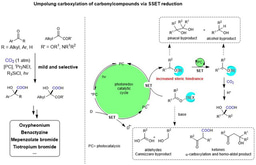
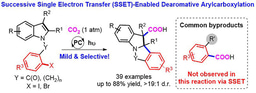
Different from the two-electron activation mode of direct attack on CO2 by nucleophile reagents,13-15 the single-electron reduction of CO2 into CO2 radical anion (CO2•−), which has unique reaction intermediates and reaction pathways, can achieve diverse carboxylation reactions with different chemo- and regio-selectivity, thus providing a new method for synthesis of important carboxylic acid compounds.16 However, because of the high reduction potential of CO2 [E1/2 (CO2/CO2•−) = -2.21V vs SCE]17 and the limited energy of a single visible-light photon,18 it is hard to directly reduce CO2 by conventional visible-light photocatalytic system, resulting in very limited reports in such fields. In addition, prior to this work, there is no report of CO2•− attacking unactivated alkene under visible-light irradiation. In view of the above challenges, the research group of Prof. Dr. Da-Gang Yu at Sichuan University (China), which mainly focuses on CO2 utilization and transformation (Research summary: Acc. Chem. Res. 2021, 54, 2518; Selected research works: Angew. Chem. Int. Ed. 2017, 56, 15416; Angew. Chem. Int. Ed. 2018, 57, 13897; J. Am. Chem. Soc. 2018, 140, 17338; Nat. Commun. 2019, 10, 3592; Angew. Chem. Int. Ed. 2020, 59, 21121; Nat. Commun. 2020, 11, 3263; Nat. Catal. 2021, 4, 304; J. Am. Chem. Soc. 2021, 143, 2812; Nat. Commun. 2021, 12, 3306; Chem 2021, 7, 3099; CCS Chem. 2021, 3, 1746; Sci. China Chem. 2021, 64, 1164; Chin. J. Catal. 2022, 43, 1667; Chin. J. Catal. 2022, 43, 2388; ACS Catal. 2022, 12, 18.) reported the visible-light photocatalyzed di- and hydro-carboxylation of unactivated alkenes with CO2. In this work, the consecutive photo-induced electron transfer (ConPET) strategy, which can produce strong reductive photosensitizers, is used to reduce CO2 into CO2•− which then attack unactivated alkene to deliver corresponding alkyl radicals. The alkyl radicals could undergo intermolecular hydrogen atom transfer (HAT) process to generate selective hydro-carboxylation products. What’s more, the alkyl radical could also undergo intramolecular HAT when the substrates are reasonably designed to generate more stable benzyl radicals, which further underwent single atom transfer (SET) process to form benzyl carbanion. Finally, its nucleophilic attack on CO2 and following protonation can provide the corresponding di-carboxylation products. Mechanistic experimental results confirmed the related intermediates and ConPET. Importantly, a series of alkyl alkenes, including propylene and mixed alkenes from industrial sources, could proceed well in this reaction, yielding diverse monocarboxylic acid, dicarboxylic acid and unnatural α-amino acid derivatives in moderate-to-good yields.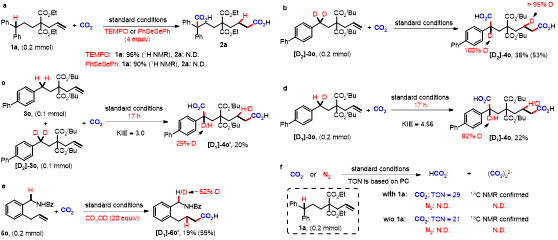
More details of this work could be found here: “Visible-Light Photocatalytic Di- and Hydro-Carboxylation of Unactivated Alkenes with CO2” in Nature Catalysis.
References
- Ciamician, G. The photochemistry of the future. Science 36, 385–394 (1912)
- Grignard, B., Gennen, S., Jérôme, C., Kleij, A. W. & Detrembleur, C. Advances in the use of CO2as a renewable feedstock for the synthesis of polymers. Soc. Rev. 48, 4466-4514 (2019).
- Stephenson, C., Yoon, T. & MacMillan, D. W. C. Visible Light Photocatalysis in Organic Chemistry (Wiley-VCH, 2018).
- Goddard, J.-P., Ollivier, C. & Fensterbank, L. Photoredox catalysis for the generation of carbon centered radicals. Acc. Chem. Res. 49, 1924–1936 (2016).
- Cao, Y., He, X., Wang, N., Li, H.-R. & He, L.-N. Photochemical and Electrochemical Carbon Dioxide Utilization with Organic Compounds. J. Chem. 36, 644-659 (2018).
- Yeung, C. S. Photoredox Catalysis as a Strategy for CO2Incorporation: Direct Access to Carboxylic Acids from a Renewable Feedstock. Chem. Int. Ed. 58, 5492-5502 (2019).
- Cai, B., Cheo, H. W., Liu, T. & Wu, J. Light-Promoted Organic Transformations Utilizing Carbon-Based Gas Molecules as Feedstocks. Chem. Int. Ed. 60, 2-33 (2021).
- Ye, J.-H. Ju, T. Huang, H. Liao, L.-L. & Yu, D.-G. Radical Carboxylative Cyclizations and Carboxylations with CO2. Chem. Res. 54,2518-2531 (2021).
- Yatham, V. R., Shen, Y. & Martin, R. Catalytic Intermolecular Dicarbofunctionalizationof Styrenes with CO2 and Radical Precursors. Chem. Int. Ed. 56, 10915-10919 (2017).
- Ye, J.-H. et al. Visible-Light-Driven Iron-Promoted Thiocarboxylation of Styrenes and Acrylates with CO2. Chem. Int. Ed. 56, 15416-15420 (2017).
- Gaydou, M., Moragas, T., Juliá-Hernández, F. & Martin, R. Site-Selective Catalytic Carboxylation of Unsaturated Hydrocarbons with CO2and Water. Am. Chem. Soc. 139, 12161-12164 (2017).
- Song, L. et al. Visible-Light Photoredox-Catalyzed Remote Difunctionalizing Carboxylation of Unactivated Alkenes with CO2. Chem. Int. Ed. 59, 21121-21128 (2020).
- Cao, Y., He, X., Wang, N., Li, H.-R. & He, L.-N. Photochemical and electrochemical carbon dioxide utilization with organic compounds. J. Chem. 36, 644–659 (2018).
- Yeung, C. S. Photoredox catalysis as a strategy for CO2incorporation: direct access to carboxylic acids from a renewable feedstock. Chem. Int. Ed. 58, 5492–5502 (2019).
- Cai, B., Cheo, H. W., Liu, T. & Wu, J. Light-promoted organic transformations utilizing carbon-based gas molecules as feedstocks. Chem. Int. Ed. 60, 2–33 (2021).
- Matthessen, R., Fransaer, J., Binnemans, K. & Vos, D. E. D. Electrocarboxylation: towards sustainable and efficient synthesis of valuable carboxylic acids. Beilstein Org. Chem. 10, 2484–2500 (2014).
- Rosso, J. A., Bertolotti, S. G., Braun, A. M., Mártire, D. O. & Gonzalez, M. C. Reactions of carbon dioxide radical anion with substituted benzenes. Phys. Org. Chem. 14, 300-309 (2001).
- Cybularczyk-Cecotka, M., Szczepanik, J. & Giedyk, M. Photocatalytic strategies for the activation of organic chlorides. Catal.3, 872–886 (2020).
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in