Visible-light photoredox-catalyzed umpolung carboxylation of carbonyl compounds with CO2
Published in Chemistry

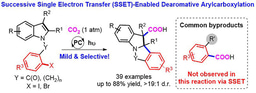
The research group of Prof. Da-Gang Yu at Sichuan University has been focusing on the development of strategies for CO2 utilization and visible-light photoredox catalysis in the past few years. In the area of radical carboxylative cyclizations and carboxylations with CO2 (key review: Acc. Chem. Res. 2021, 54, 2518.), we have chieved visible light-driven difluoroalkylation and alkylation of allylamines (OL 2017, 20, 190; OL 2018, 20, 3049), difunctionalization carboxylation of π system (ACIE 2017, 56, 15416; Nat. Commun. 2019, 10, 3592; Nat. Commun. 2020, 11, 3263; ACIE 2020, 59, 21121; JACS 2021, 143, 2812; Nat. Catal. 2021, 4, 304; SCC. 2021, https://doi.org/10.1007/s11426-021-1004-y) and anti-markovnikov hydrocarboxylation of alkene (CCS Chem. 2020, 2, 1746). Also, we have achieved the selective umpolung carboxylations of imines, enamides, tetraalkylammonium salts, and oxime esters via successive single-electron-transfer (SSET) reduction (ACIE 2018, 57, 13897; JACS 2018, 140, 17338; ChemSusChem 2020, 13, 6312). In our recent paper published in Nature Communications, we described the latest progress on this topic. Developing a general strategy for reductive carboxylation of carbonyl compounds with CO2 to give valuable alfa-hydroxycarboxylic acids, which are key intermediates to produce many drugs and natural products, including oxyphe-onium, mepenzolate bromide, benactyzine, and tiotropiumas.
Carbonyl compond are important bulk chemicals in industry and they exist widely in natural products, pharmaceuticals, and materials. Recently, visible light-mediated SET umpolung reaction of carbonyl group has received intensive attention owing to their mild reaction conditions and green processes1,2. In particular, photocatalytic proton-coupled electron transfer (PCET)3 has evoked a new round of exploration on carbonyl chemistry, which can achieve ketyl−olefin coupling and radical-radical coupling by generating ketyl radical. However, the direct radical addition of ketyl radicals to CO2 is less favored4, a second SET reduction to generate a carbanion, which could attack electrophilic CO2, would be promising. However, achieving such a goal requires avoiding common side reactions, namely pinacol coupling5 and hydrogen atom transfer (HAT)6 of ketyl radicals, direct a-carboxylation, homo-aldol and Cannizzaro reaction under basic conditions. With such challenges in mind, we were inspired by PCET and hypothesized whether we could use a Lewis acidic chlorosilane instead of a proton as an activating group to promote the SET reduction of a carbonyl group. Moreover, the activating chlorosilane might act as a temporary protecting group to generate alfa-silyloxy carbon radicals, which would increase the steric hindrance of the radical intermediates and thus retard undesired pinacol coupling. Based on this design, we developed a general and practical method for the carboxylation of diverse carbonyl compounds, including alkyl aryl ketones, diaryl ketones, alfa-ketoamides, alfa-ketoesters, and aryl aldehydes, to give valuable alfa-hydroxycarboxylic acids. And control experiments demonstrated the important role of chlorosilanes to promote the reaction and carbon radicals and carbanions might be involved in this transformation.
More details of this work could be found here: “ Visible-light photoredox-catalyzed umpolung carboxylation of carbonyl compounds with CO2” in Nature Communications (https://www.nature.com/articles/s41467-021-23447-8).
References
- Lee, K. N. & Ngai, M.-Y. Recent developments in transition-metal photoredox-catalysed reactions of carbonyl derivatives. Chem. Commun. 53, 13093-13112 (2017).
- Xia, Q., Dong, J., Song, H. & Wang, Q. Visible-Light Photocatalysis of the Ketyl Radical Coupling Reaction. Chem. Eur. J. 25, 2949-2961 (2019).
- Gentry, E. C. & Knowles, R. R. Synthetic Applications of Proton-Coupled Electron Transfer. Acc. Chem. Res. 49, 1546-1556 (2016).
- Isse, A. A. & Gennaro, A. Mechanism of the Electrochemical Carboxylation of Aromatic Ketones in Dimethylformamide. Collect. Czech. Chem. Commun. 68, 1379-1394 (2003).
- Nakajima, M., Fava, E., Loescher, S., Jiang, Z. & Rueping, M. Photoredox-Catalyzed Reductive Coupling of Aldehydes, Ketones, and Imines with Visible Light. Angew. Chem. Int. Ed. 54, 8828-8832 (2015).
- Ishitani, O., Yanagida, S., Takamuku, S. & Pac, C. Redox-photosensitized reactions. Ru(bpy)32+-photosensitized reactions of an NADH model, 1-benzyl-1,4-dihydronicotinamide, with aromatic carbonyl compounds and comparison with thermal reactions. J. Org. Chem. 52, 2790-2796 (1987).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in