Reductive Dearomative Arylcarboxylation via Visible-Light Photoredox Catalysis
Published in Chemistry

The research group of Prof. Da-Gang Yu at Sichuan University has been focusing on developing new strategies for CO2 utilization and visible-light photoredox catalysis in the past 5 years. In the area of visible-light-driven CO2 utilization, we have developed the visible-light-driven difluoroalkylation and alkylation of allylamines to synthesize oxazolinones (OL 2017, 20, 190; OL 2018, 20, 3049), the thiocarboxylation of alkenes with unique regioselectivity (ACIE 2017, 56, 15416), the reductive hydrocarboxylation of enamides/imines (ACIE 2018, 57, 13897), umpolung carboxylation of C-N bonds in the tetraalkyl ammonium salts (JACS 2018, 140, 17338) as well as redox-neutral phosphonocarboxylation of alkenes with CO2 (Nat. Commun. 2019, 10, 3592). Very recently, we have developed a novel strategy for reductive dearomative arylcarboxylation of indoles with CO2 via visible-light photoredox catalysis, as shown below.
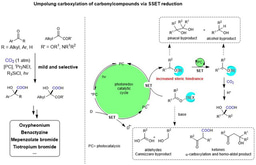
Tandem reductive cyclization/cross coupling of unsaturated bond has been an important tool in organic synthesis.1 Conventional transition metal (TM)-catalyzed reductive coupling is powerful protocol in tuning the reactivity and selectivity. However, air- and moisture-sensitive organometallic reagents and unavoidable side reaction remain unsolved challenge.2 On the other hand, dearomatization of indoles is an efficient method to provide 3D cyclic skeletons indolines, which exist widely in pharmaceuticals and bioactive natural products.3 Although many methods have been developed in this field,4-9 the dearomative reductive coupling of indoles with two electrophiles is still challenging due to the stability of carbon-carbon double bond within aromaticity and thus slow migratory insertion rate. With these challenges in mind, we envisioned and successfully realized such reductive dearomative difunctionalization via radical pathway with successive single electron transfer (SSET) process. Notably, this reaction is highly chemoselective, as common side reactions in transition metal catalysis, including ipso-carboxylation of aryl halides and β-hydride elimination, are avoided. This method also shows high selectivity, low loading of photocatalyst, generally good yields, mild reaction conditions (room temperature, 1 atm), good functional group tolerance and broad substrate scope, providing great potential for the synthesis of valuable but difficultly accessible indoline-3-carboxylic acids. Mechanistic studies indicate that the benzylic radicals and anions might be generated as the key intermediates. In addition, gram-scale reaction and facile derivatizations have been demonstrated that the utility of this strategy.
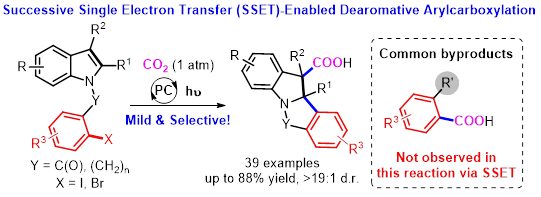
In conclusion, the successive single electron transfer (SSET) strategy not only provides a direction for dearomative difunctionalization but also represents a strategy to realize selective tandem reductive cyclization/cross couplings, preventing the undesired two-component couplings and reductive Heck-type reactions via β-hydride elimination. More details of this work can be found here: “Reductive Dearomative Arylcarboxylation of Indoles with CO2 via Visible-Light Photoredox Catalysis” in Nature Communications.
References
- Wang, K., Ding, Z., Zhou, Z. & Kong, W. Ni-Catalyzed Enantioselective Reductive Diarylation of Activated Alkenes by Domino Cyclization/Cross-Coupling. J. Am. Chem. Soc. 140, 12364–12368 (2018).
- Li, X. et al. Palladium-Catalyzed Enantioselective Intramolecular Dearomative Heck Reaction. J. Am. Chem. Soc. 140, 13945–13951 (2018).
- Xia, Z. , Xu, Q. F., Zheng, C. & You, S.-L. Chiral Phosphoric Acid-Catalyzed Asymmetric Dearomatization Reactions. Chem. Soc. Rev. 49, 286-300 (2020).
- Liu, R. R. et al. Enantioselective Dearomative Difunctionalization of Indoles by Palladium-Catalyzed Heck/Sonogashira Sequence. Angew. Chem. Int. Ed. 56, 7475–7478 (2017).
- Marchese, A. D., Lind, F., Mahon, Á. E., Yoon, H. & Lautens, M. Forming Benzylic Iodides via a Nickel Catalyzed Diastereoselective Dearomative Carboiodination Reaction of Indoles. Angew. Chem. Int. Ed. 58, 5095–5099 (2019).
- Shen, C. et al. Enantioselective arylative dearomatization of indoles via Pd-catalyzed intramolecular reductive heck reactions. J. Am. Chem. Soc. 137, 4936–4939 (2015).
- Qin, X., Lee, M. W. Y. & Zhou, J. S. Nickel-Catalyzed Asymmetric Reductive Heck Cyclization of Aryl Halides to Afford Indolines. Angew. Chem. Int. Ed. 56, 12723–12726 (2017).
- Zeidan, N., Beisel, T., Ross, R. & Lautens, M. Palladium-Catalyzed Arylation/Heteroarylation of Indoles: Access to 2,3-Functionalized Indolines. Org. Lett. 20, 7332–7335 (2018).
- Li, X. et al. Palladium-Catalyzed Enantioselective Intramolecular Dearomative Heck Reaction. J. Am. Chem. Soc. 140, 13945–13951 (2018).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in