Nickel-catalyzed electrochemical carboxylation of unactivated aryl and alkyl halides with CO2
Published in Chemistry

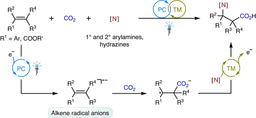
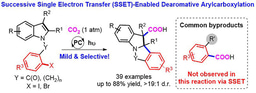
The research group led by Prof. Da-Gang Yu at Sichuan University has been focusing on developing new strategies for CO2 utilization in the past few years (key review: Acc. Chem. Res. 2021, 54, 2518). Since organic electrochemistry has shown great power in activating the inert molecules, we believe that it would also be an effective tool for CO2 fixation. In our recent paper published on Nature Communication, we described the latest progress on the topic of electrochemical carboxylation. A general strategy is developed for electrochemical carboxylation of unactivated aryl and alkyl halides with CO2 to give the valuable aryl and alkyl acids, which would be the key intermediates for the construction of many drugs and natural products, as shown below.
Carboxylation of organohalides with CO2 is a direct and effective way to construct valuable carboxylic acids due to the abundant, nontoxic and recyclable properties of CO21,2. However, the thermodynamic stability and kinetic inertness make it difficult to achieve high efficiency under mild reaction conditions. It is even more challenging when the inexpensive coupling partners are less reactive. Although recent progress has shown some robust catalytic manners in transition-metal catalysis3 and photocatalysis4,5, the use of stoichiometric amount of extra metallic reductants, expensive Ir-photosensitizer or reactive organohalides still limits their wide application.
Organic electrochemistry has shown significant progress in the recent years6. The redox processes could be tuned by external DC power supply with electron serving as the clean redox reagent, which would be an ideal manner for achieving the electroreductive carboxylation. Early studies on the electrochemical carboxylation of activated oganic (pseudo) halides have been investigated7,8. However, selective electrocatalytic carboxylation of unactivated aryl chlorides and unactivated alkyl halides with CO2 has rarely been realized9,10, which is more challenging due to the low reactivity of both unactivated organohalides and CO2 as well as many competing side reactions, such as protonation, β-H elimination, migration and homodimerization of the generated organometallic reagents and electrochemical decarboxylation of the products11. Besides the limitations in substrate scope, sacrificial anodes are commonly used in most of electroreductive carboxylation, which is not practicle and sustainable for its application12. With these issues in mind, we envisioned and successfully realized the electrocatalytic carboxylation of challenging aryl chlorides and unactivated alkyl bromides with CO2 via nickel catalysis. The desired reaction takes place in an efficient manner, showing high chemoselectivity, wider substrates scope, functional groups tolerance and mild conditions, which provides great potential for the synthesis of valuable carboxylic acids. In addition, we also realize the catalytic electrochemical carboxylation of aryl (pseudo)halides with CO2 avoiding the use of sacrificial electrodes. Mechanistic investigations indicate that the reaction might proceed via oxidative addition of aryl halides to Ni(0) complex, the reduction of aryl-Ni(II) adduct to the Ni(I) species and the following carboxylation with CO2.
More details of this work can be found here: "Nickel-Catalyzed Electrochemical Carboxylation of Unactivated Aryl and Alkyl Halides with CO2" in Nature communication (https://www.nature.com/articles/s41467-021-27437-8).
Reference
- Ackermann, L. Transition-Metal-Catalyzed Carboxylation of C-H Bonds. Angew. Chem. Int. Ed. 50, 3842-3844 (2011).
- Huang, K., Sun, C.-L. & Shi, Z.-J. Transition-metal-catalyzed C–C bond formation through the fixation of carbon dioxide. Chem. Soc. Rev. 40, 2435-2452 (2011).
- Tortajada, A., Juliá-Hernández, F., Börjesson, M., Moragas, T. & Martin, R. Transition-Metal-Catalyzed Carboxylation Reactions with Carbon Dioxide. Angew. Chem. Int. Ed. 57, 15948-15982 (2018).
- He, X., Qiu, L.-Q., Wang, W.-J., Chen, K.-H. & He, L.-N. Photocarboxylation with CO2: an appealing and sustainable strategy for CO2 fixation. Green Chem. 22, 7301-7320 (2020).
- Zhang, Z. et al. Visible-Light-Driven Catalytic Reductive Carboxylation with CO2. ACS Catal. 10, 10871-10885 (2020).
- Yan, M.; Kawamata, Y. & Baran, P. S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 117, 13230-13319 (2017).
- Senboku, H. & Katayama, A. Electrochemical carboxylation with carbon dioxide. Curr. Opin. Green Sustainable Chem. 3, 50-54 (2017).
- Jiao, K.-J. et al. Palladium-catalyzed reductive electrocarboxylation of allyl esters with carbon dioxide. Org. Chem. Front. 5, 2244-2248 (2018).
- Yang, H. P. et al. A Core-Shell-Structured Silver Nanowire/Nitrogen-Doped Carbon Catalyst for Enhanced and Multifunctional Electrofixation of CO2. ChemSusChem 11, 3905-3910 (2018).
- Bazzi, S., Le Duc, G., Schulz, E., Gosmini, C. & Mellah, M. CO2 activation by electrogenerated divalent samarium for aryl halide carboxylation. Org. Biomol. Chem. 17, 8546-8550 (2019).
- Shi, S.-H., Liang, Y. & Jiao, N. Electrochemical Oxidation Induced Selective C–C Bond Cleavage. Chem. Rev. 121, 485-505 (2021).
- Matthessen, R., Fransaer, J., Binnemans, K. & De Vos, D. E. Electrocarboxylation: towards sustainable and efficient synthesis of valuable carboxylic acids. Beilstein J. Org. Chem. 10, 2484-2500 (2014).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in