Towards Automatic Home-Based Sleep Apnea Estimation Using Deep Learning
Published in Neuroscience, Computational Sciences, and General & Internal Medicine

Sleep apnea is a prevalent sleep disorder characterized by repeated interruptions in breathing that affects a significant portion of the adult population. These interruptions, known as apnea and hypopnea events, can lead to serious health issues such as cardiovascular disease, diabetes, and cognitive impairments. The Apnea-Hypopnea Index (AHI), defined as the count of apneas and hypopneas per hour of sleep, is used to diagnose patient’s severity. Traditionally, this index is determined by an overnight sleep study called polysomnography (PSG), conducted in specialized sleep clinics. However, PSG is costly, inconvenient, and often inaccessible, resulting in a high rate of undiagnosed cases.
DRIVEN can help to automatically diagnose sleep apnea and segment sleep studies at home
Our study proposes an innovative solution named DRIVEN, which leverages deep learning to estimate the AHI using data from at-home wearable devices, offering a more comfortable and cost-effective alternative to sleep studies at the clinic. DRIVEN (Deep-learning-based RecognitIon of Sleep Apnea eVENts) is designed to automatically estimate AHI and detect apnea, hypopnea, and wakefulness events during sleep. Patients can wear a combination of sensors, measuring abdominal and thoracic movements and pulse oximetry, that are easy to use at home. The method utilizes deep convolutional neural networks (CNNs) to process the physiological signals and a light gradient-boost machine (LightGBM) for event classification (Figure 1).
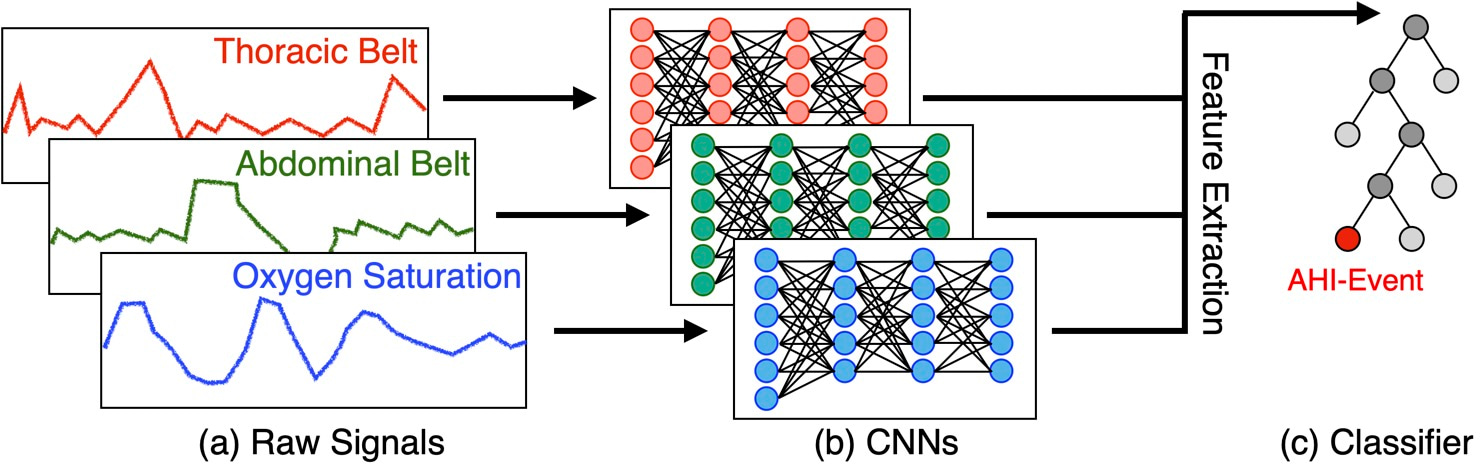
Our study employs publicly available data from three large sleep studies: the Multi-Ethnic Study of Atherosclerosis (MESA), the Men Study of Osteoporotic Fracture (MrOS), and the Sleep Heart Health Research (SHHS). These datasets comprise over 14,000 PSG recordings from diverse populations, providing a robust foundation for training and validating the DRIVEN model. We used the SHHS dataset for training and validation, and tested our model in the MESA and MrOS datasets.
To prepare the data, we normalized the physiological signals to a uniform sampling frequency and segmented the whole overnight studies into 30-second windows. Each segment was labeled as either normal or an AHI-event (apnea or hypopnea). The CNNs were trained to extract features from these segments, which were then fed into the LightGBM classifier to determine the presence of AHI-events. This hybrid approach leverages the strengths of both deep learning and gradient-boosted trees, resulting in a highly accurate and generalizable model. To be able to determine AHI, the algortihm also needs to predict the sleeping segments of the paper. For this, we trained the same architectute to predict if in the 30 second windows the patient is awake or sleep.
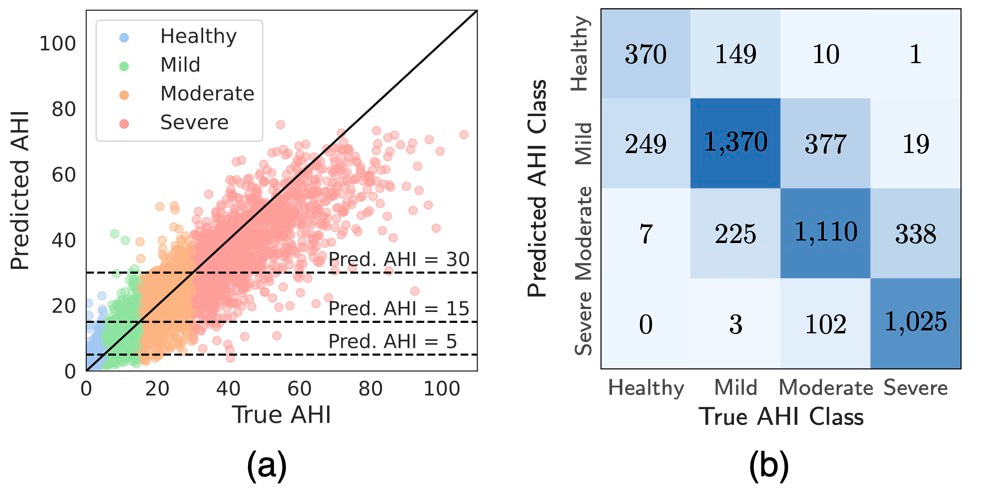
DRIVEN's performance was evaluated on both external test datasets for sleep prediction and AHI-event classification. Using only abdominal movement and pulse oximetry sensors, the model correctly classified 72.4% of patients into the correct AHI severity category, with 99.3% either correctly classified or placed within one category of the true class (Figure 2). This level of accuracy, achieved with minimal sensors, represents a reasonable trade-off between model performance and patient comfort. The inclusion of pulse oximetry significantly enhanced performance, as it provides critical information on blood oxygen levels, a key indicator of sleep apnea events.
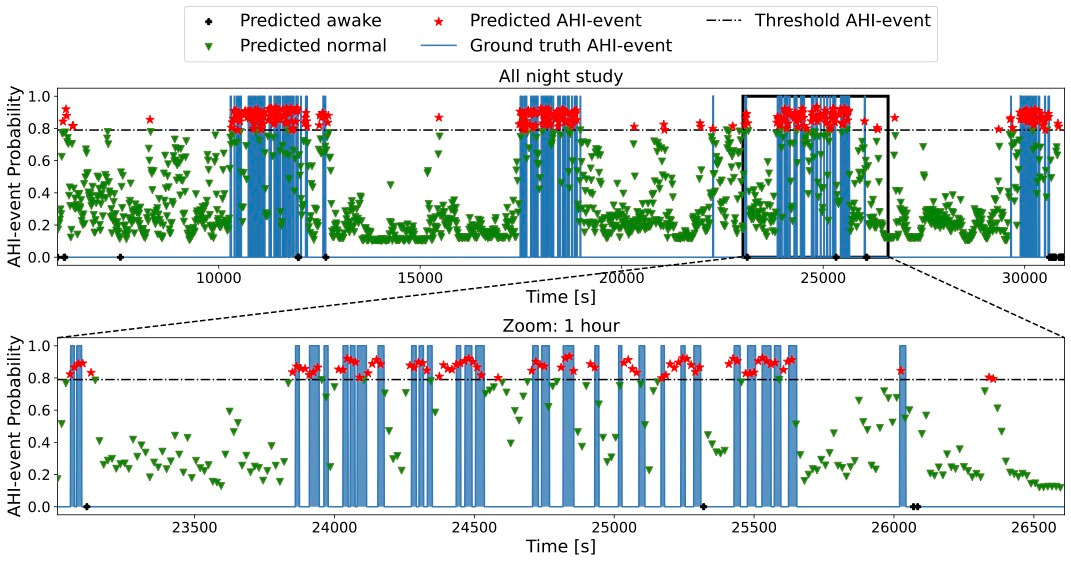
Finally, DRIVEN segmented the sleep data, highlighting the timing of AHI-events throughout the night and when the patient was sleeping and awake (Figure 3).
By enabling reliable AHI estimation at home, DRIVEN addresses the limitations of traditional PSG, offering a more accessible and comfortable solution for patients. This innovation can reduce the burden on healthcare systems, improve patient care, and facilitate early detection and treatment of sleep apnea.
For healthcare providers, DRIVEN provides a valuable tool to assist in the diagnosis and management of sleep apnea. The ability to monitor patients over extended periods in their natural sleep environment can lead to more accurate assessments and personalized treatment plans.
While DRIVEN demonstrates promising results, there are areas for further improvement. Future research could explore the integration of additional sensors, such as accelerometers or smartwatches, to enhance sleep state detection and AHI estimation. Moreover, advancements in AI models, including selective state-space neural networks, could further refine the accuracy and robustness of the method.
In conclusion, DRIVEN represents a significant step towards reliable long-term sleep monitoring at home. This could lead to earlier interventions, better health outcomes, and a reduction in the economic burden of untreated sleep apnea. By leveraging the power of deep learning, DRIVEN offers a transformative approach to sleep apnea diagnosis, providing more accessible and effective patient care.
References:
Senaratna, C. V. et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Medicine Reviews 34, 70–81 (2017).
Riha, R. L. Defining obstructive sleep apnea syndrome: a failure of semantic rules. Breathe 17, 210082 (2021).
Tan, M. & Le, Q. EfficientNetV2: Smaller models and faster training. In International Conference on Machine Learning, 10096–10106 (PMLR, 2021).
Ke, G. et al. Lightgbm: A highly efficient gradient boosting decision tree. Advances In Neural Information Processing Systems 30 (2017).
Chen, X. et al. Racial/ethnic differences in sleep disturbances: the multi-ethnic study of atherosclerosis (mesa). Sleep 38, 877–888 (2015).
Blackwell, T. et al. Associations between sleep architecture and sleep disordered breathing and cognition in older community-dwelling men: the osteoporotic fractures in men sleep study. Journal of The American Geriatrics Society 59, 2217–2225 (2011).
Quan, S. F. et al. The sleep heart health study: design, rationale, and methods. Sleep 20, 1077–1085 (1997).
Gu, A. & Dao, T. Mamba: Linear-Time Sequence Modeling with Selective State Spaces. Preprint at arXiv:2312.00752 (2023).
Follow the Topic
-
npj Digital Medicine

An online open-access journal dedicated to publishing research in all aspects of digital medicine, including the clinical application and implementation of digital and mobile technologies, virtual healthcare, and novel applications of artificial intelligence and informatics.
Related Collections
With Collections, you can get published faster and increase your visibility.
Digital Health Equity and Access
Publishing Model: Open Access
Deadline: Mar 03, 2026
Evaluating the Real-World Clinical Performance of AI
Publishing Model: Open Access
Deadline: Jun 03, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in