When a Forgotten Experiment Revealed the Secret Life of Solids
Published in Chemistry, Materials, and Sustainability
Metal halide perovskites are “rock stars” of modern materials science. These crystalline compounds, named after a 19th-century Russian mineralogist and nobleman, can convert sunlight into electricity, emit a broad range of colors, and even sense high-energy radiation. Yet their traditional synthetic approaches often require undesirable hazardous solvents, while solvent-free methodologies usually leave scientists blind to how these materials form. Can we synthesize perovskites without solvents and watch the evolution of their functional properties in real time?
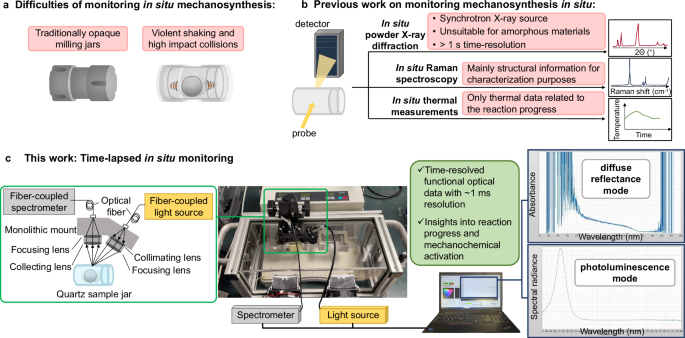
Building a Window into Chemistry: The Time-Lapsed In Situ (TLIS) Spectrometer
The answer came from a marriage of old and new. Mechanochemistry is a process that underpins the formation of the earth’s crust and involves grinding solids to trigger reactions1,2. Here, we gave it a 21st-century twist by swapping opaque milling jars for transparent quartz and installing a customized optical spectrometer, we can (literally) shine light into the reaction (Fig. 1a). Our TLIS spectrometer is essentially a high-speed camera for materials and can track the changes in light absorption or emission every millisecond. This lets us observe the evolution of chaotic precursors into ordered perovskite crystals3.
.jpg)
Fig. 1 | Schematic illustration of the TLIS spectrometer and its applications in diffuse reflectance and photoluminescence analyses. a Experimental setup comprising a light source, spectrometer, and computer interface for data acquisition. The system operates in two measurement modes: diffuse reflectance and photoluminescence. b Diffuse reflectance mode for monitoring phase transitions of FAPbI3 over time, showing absorbance spectra from the α-phase to δ-phase with time-resolved spectral evolution. c Photoluminescence mode demonstrating the spectral radiance of Cs2Na0.9Ag0.1BiCl6, highlighting PL intensity changes due to ball milling and storage time, accompanied by Cl- ion migration mechanisms.
Saving the “Black Phase” of Tecnologically Relevant Perovskites from Collapse
Lead-based perovskites like formamidinium lead triiodide (FAPbI3) are solar cell superstars, but they’re tragically unstable. Their prized “black” phase (α-FAPbI3) transforms into a yellow form (δ-FAPbI3) that is ineffective for light harvesting at room temperature. With our TLIS spectrometer, we witnessed this decomposition live: after 48 minutes of ball milling, the light absorption peaked—only to decay as the α-FAPbI3 underwent further phase transitions (Fig. 1b).
Adding just 20 mol% of methylammonium (MA+) helped to stabilize the black phase. The TLIS of the solid-state ball milling reaction allowed us to model and quantify the kinetics live: the phase transition slowed by 3.5-fold with MA+.
The Case of the Self-Improving Perovskite
While studying a lead-free double perovskite previously shown to emit broadband white photoluminescence (PL) with respectable photoluminescence quantum yields, Cs2Na0.9Ag0.1BiCl6, we ball milled the precursor salts for six hours on a Thursday and saw zero PL. Frustrated and slightly doubtful of previous reports, we tried three more hours of ball milling on Friday but still observed minimal PL. But science loves accidents. Distracted by weekend plans, I left the sample in the jar under a blanket of inert nitrogen gas. On Monday, when I started up the TLIS spectrometer without any ball milling, a surprising but tell-tale glow appeared at 680 nm. The inactive perovskite had come to life with the PL intensity surging 106-fold over 45 hours of storage at room temperature (Fig. 1c). Was it a gas leak? Probably not, since the jar was sealed under nitrogen gas and oxygen will typically quench instead of enhancing PL. Light exposure? Unlikely, since the samples were stored under a dark cloth. Crystal structure changes? The X-ray diffraction patterns showed ostensibly identical features after six hours of ball milling and after three days of storage. Was this reproducible? Definitely, and for more than one composition of lead-free double perovskite.
The invisible phenomenon? Chloride ions migrating silently through the solid, healing defects and growing larger nanocrystalline domains. This previously unreported “self-healing” behavior was captured by our TLIS optical and confirmed by solid-state NMR spectroscopy, transmission electron microscopy, and density functional theory calculations.
Turning Tin’s Weakness into Strength
Tin-based perovskites have lower environmental impact than their lead-based congeners but are notoriously fragile since the tin(II) ions rapidly in air. By blending bromine and iodine into formamidinium tin halides (FASnI2.5Br0.5), we tuned their emission to the near-infrared wavelengths, which are ideal for biomedical imaging. However, the instability lingered. We strengthened the perovskite with a chloride shell to form FASnCl3. The TLIS emission data revealed a brighter PL and greater resistance to oxidation, while scanning transmission electron microscopy confirmed that a protective shell had formed at the particle boundaries (Fig. 2).

Fig. 2 | TLIS PL improvements and stabilization of NIR-emissive FASnI2.5Br0.5. (a) TLIS PL spectra, (b) STEM images and the STEM energy dispersive EDS maps of FASnI2.5Br0.5@FASnCl3 after 1:1 FACl and SnCl2 was added under N2 to the mechanosynthesized FASnI2.5Br0.5. The dark-field image of a particle and the I (red), Cl (blue), and co-localized I and Cl EDS maps are shown.
Why TLIS Optical Spectroscopy During Mechanochemistry Matters?
Mechanochemical synthesis eliminates hazardous solvents, which aligns with multiple global sustainability goals. By offering a window into the evolution of the light absorption and PL properties of materials, our TLIS spectrometer can cut down the time needed for the optimization of optoelectronic materials from months to days, or even hours. Our TLIS “camera” can of course also be applied to other materials, such as quantum dots, catalysts, pigments, cosmetics, and even food coloring, to name a few applications.
Reference
1. Hong, Z., Tan, D., John, R. A., Tay, Y. K. E., Ho, Y. K. T., Zhao, X., Sum, T. C., Mathews, N., García, F. & Soo, H. S. Completely Solvent-free Protocols to Access Phase-Pure, Metastable Metal Halide Perovskites and Functional Photodetectors from the Precursor Salts. iScience 16, 312-325 (2019).
2. Xiao, Y. et al. Machine‐Learning‐Assisted Discovery of Mechanosynthesized Lead‐Free Metal Halide Perovskites for the Oxidative Photocatalytic Cleavage of Alkenes. Adv. Sci. 11, 2309714 (2024).
3. Xiao, Y. et al. Optical time-lapsed in situ mechanochemical studies on metal halide perovskite systems. Nat. Commun. 16, 1362 (2025).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
What are SDG Topics?
An introduction to Sustainable Development Goals (SDGs) Topics and their role in highlighting sustainable development research.
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in