Why would tumor-associated Tregs overexpress the MHC-II invariant chain ?
Published in Immunology
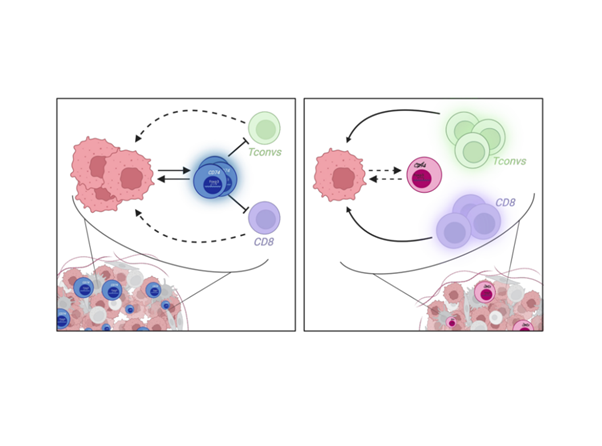
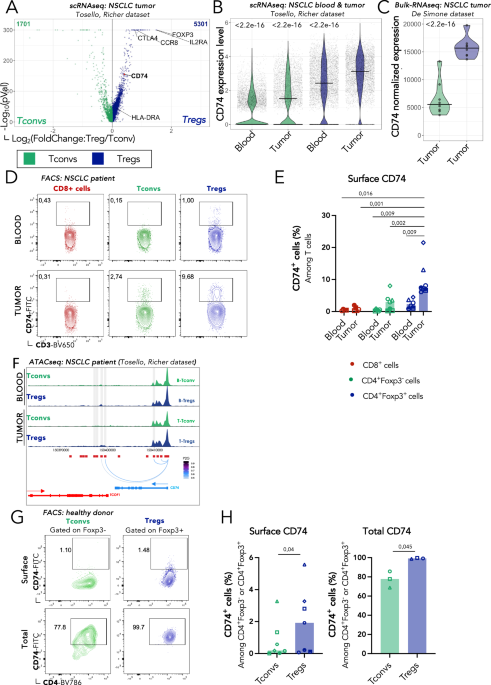
As all well-engineered processes, the immune system relies on regulators essential to maintain tolerance, limit strong inflammation and restore steadiness. The concept of a subset of CD4+ T cells with suppressive capacities was first suggested in the 1980s, and then reinforced in 1995, with the description of precise characteristics of this suppressive subset enabling the identification of what is known today as regulatory T cells (Tregs) (Ref.1). Now, it is well known that Tregs are key players of immune modulation. They constitute a heterogeneous and plastic population able to develop optimized mechanisms to efficiently regulate immune responses according to the tissue in which they reside. Nonetheless, in some pathologies, Treg functions represent limits for a competent immunity. Treg adaptation to external cues can induce Treg instability associated with a switch from suppressive to pro-inflammatory functions, leading to the development of autoimmunity, or alternatively, associated with a reinforcement of their regulatory mechanisms, promoting anti-tumor response (Ref.2-3). Thus, characterizing Treg features associated to individual tissues or pathologies should help in the design of optimized immunomodulatory therapies. For cancer application, one of the finest strategies would be to inhibit Treg function only in the tumor, while sparing Tregs in the rest of the body. To this end, tumor-associated Treg (tumTreg) traits are being investigated, and today the identification and characterization of tumTreg unique biomarkers remain understudied (Ref.4-5). Using bulk RNA, single-cell RNA and TCR sequencing data, we observed that tumTregs selectively overexpress CD74, the MHC-II invariant chain. In this work, we aimed to evaluate the role of CD74 in Treg biology to bring light in tumor-specific Treg mechanisms (Ref.6-7-8).
First, we validated at the protein level that tumTregs express higher levels of the CD74 protein on membrane compared to healthy donor Tregs from blood; and to CD4+ T conventional cells both from healthy donor blood and from tumors. This CD74 expression profile indicates that the induction of membrane CD74 is specific to tumTregs and that CD74 may be involved in a tumor-specific Treg function.
To investigate CD74 role in Tregs, we compared the phenotype and biological activity of CD74KO and WT Tregs in vitro and in vivo. In vitro, the survival, phenotype and suppressive capacity of Tregs were maintained in CD74KO Tregs. In vivo, in a model of xeno-GvHD, CD74KO Tregs efficiently controlled the development of the disease in a similar manner to WT Tregs, indicating that CD74 was not required for the suppressive activity of Tregs in this model.
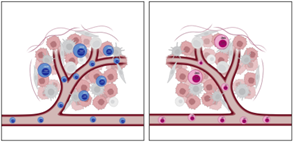
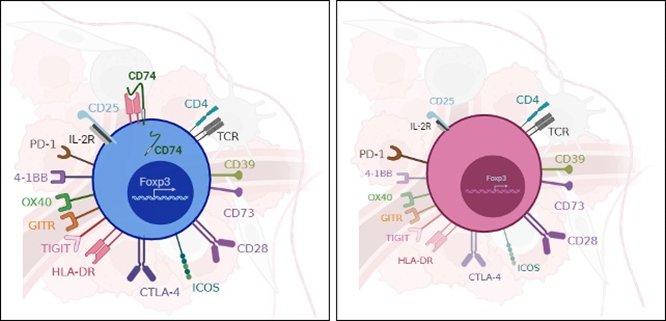
Interestingly, in a tumor model, CD74KO Tregs failed to accumulate in the tumor and to suppress anti-tumor responses compared to WT Tregs (Fig.1A). Digging into tumTreg evaluation, we demonstrated that tumor-infiltrating CD74KO Tregs had an altered phenotype, with decreased expression of activation markers, such as CD25, 4-1BB or CTLA-4, lower levels of Foxp3 and increased production of IFNγ (Fig.1B). These observations were consistent with a loss of Treg suppressive function following CD74 deletion.
|
A
|
B
|
|
Figure.1 In the tumor, CD74 deletion decrease Treg retention (A), activation and stability (B). The cells in blue represents WT Tregs and the cells in red CD74KO Tregs obtained after a CRISPR-Cas9 genetic modification. |
|
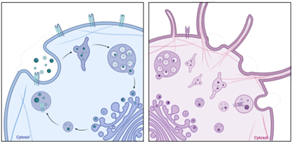
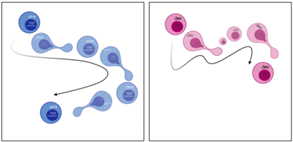
To decipher the observation made in vivo that tumTregs require CD74 to preserve their lineage characteristics and to be retained in the tumor, we performed transcriptomic analysis to compare CD74KO and WT Treg gene expression. Surprisingly, this analysis revealed that CD74 modulates structural components of Tregs, including cell cytoskeleton, Golgi apparatus, endoplasmic reticulum, and membrane, as well as RNA transcription processes. We confirmed this result with microscopy images of Tregs: compared with WT Tregs, CD74KO Tregs have a smaller size, more membrane protrusions, accumulation of early endosomes and, in general, an increased density of intracellular compartments. Consequently, the genetic deletion of CD74 in Tregs impacts their shape, membrane structure and intracellular organelles, which probably affect Treg function both intrinsically and in the interaction of Tregs with the external microenvironment and surrounding cells (Fig.2A). Finally, as the cytoskeleton is driving cellular dynamic processes, we studied the migration of Tregs in one or two dimensions using real-time imaging. Motility analyses revealed that, although CD74KO and WT Tregs spent a similar amount of time moving, CD74KO-Treg trajectory was not persistent, their speed was highly variable, and their track only covered short distances (Fig.2B). Altogether, CD74 seems to support Treg morphogenesis and migration, thus CD74 loss directly impairs mobility, velocity and cell-cell interaction of Tregs, what could explain the greater tumor infiltration and activity of WT Tregs compared to CD74KO Tregs.
|
A
|
B
|
|
Figure.2 CD74 loss impacts Treg membrane and intracellular structure (A) and altered Treg motility (B). The cells in blue represents WT Tregs and the cells in red CD74KO Tregs obtained after a CRISPR-Cas9 genetic modification. |
|
To validate our initial observation and our more advanced conclusions obtained with human cells, we set up various syngeneic models in mice. First, using mice engrafted with tumor cells, we observed that, as in humans, the surface expression of CD74 was higher in tumTregs compared to peripheral Tregs, and to tumor and peripheral CD4+ Tconvs. Second, we designed a model of adoptive cell transfer to evaluate the capacity of CD74KO and WT Treg to infiltrate the tumor in the same host mice. This second model led to a similar conclusion as in human Tregs: CD74 supports tumTreg accumulation. Finally, we generated a model with a Foxp3-specific CD74 deletion to focus on the Treg-intrinsic effects of CD74. With different tumor types, we observed that CD74-deficient Tregs showed impaired tumor infiltration and a diminution of Foxp3 expression in the tumor. The various syngeneic models reinforced the observations and hypothesis made with human Tregs, that CD74 is overexpressed by tumTregs and is a key molecule to maintain Foxp3 level in tumTregs.
In summary, we observed that, in the tumor, CD74 acts as a key molecule for Treg activation, suppressive function, identity stabilization, and infiltration. Our results reveal a novel role for CD74 in the biology of tumTregs and demonstrate the potential of CD74 as a modulator of Treg biology. Interestingly, this novel role of CD74 for Treg accumulation could be useful beyond the tumor field, as in inflammation with an opposite strategy for boosting Treg accumulation to tissues. In addition, our data suggests that CD74 plays a role in tumor adaptation of Tregs what participates in the understanding of Treg tissue-specific mechanisms.
The figures were created with BioRender.com
References
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995 Aug 1;155(3):1151-64. PMID: 7636184.
- Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, Shuai Y, Normolle DP, Kirkwood JM, Ferris RL, Delgoffe GM, Bruno TC, Workman CJ, Vignali DAA. Interferon-γ Drives Treg Fragility to Promote Anti-tumor Immunity. Cell. 2017 Jun 1;169(6):1130-1141.e11. doi: 10.1016/j.cell.2017.05.005. Epub 2017 May 25. PMID: 28552348; PMCID: PMC5509332.
- Cuadrado E, van den Biggelaar M, de Kivit S, Chen YY, Slot M, Doubal I, Meijer A, van Lier RAW, Borst J, Amsen D. Proteomic Analyses of Human Regulatory T Cells Reveal Adaptations in Signaling Pathways that Protect Cellular Identity. Immunity. 2018 May 15;48(5):1046-1059.e6. doi: 10.1016/j.immuni.2018.04.008. Epub 2018 May 8. PMID: 29752063.
- Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, Chudakov DM, Rudensky AY. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity. 2016 Nov 15;45(5):1122-1134. doi: 10.1016/j.immuni.2016.10.032. PMID: 27851913; PMCID: PMC5134901.
- De Simone M, Arrigoni A, Rossetti G, Gruarin P, Ranzani V, Politano C, Bonnal RJP, Provasi E, Sarnicola ML, Panzeri I, Moro M, Crosti M, Mazzara S, Vaira V, Bosari S, Palleschi A, Santambrogio L, Bovo G, Zucchini N, Totis M, Gianotti L, Cesana G, Perego RA, Maroni N, Pisani Ceretti A, Opocher E, De Francesco R, Geginat J, Stunnenberg HG, Abrignani S, Pagani M. Transcriptional Landscape of Human Tissue Lymphocytes Unveils Uniqueness of Tumor-Infiltrating T Regulatory Cells. Immunity. 2016 Nov 15;45(5):1135-1147. doi: 10.1016/j.immuni.2016.10.021. PMID: 27851914; PMCID: PMC5119953.
- Schröder B. The multifaceted roles of the invariant chain CD74--More than just a chaperone. Biochim Biophys Acta. 2016 Jun;1863(6 Pt A):1269-81. doi: 10.1016/j.bbamcr.2016.03.026. Epub 2016 Mar 28. PMID: 27033518.
- Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Mice lacking the MHC class II-associated invariant chain. Cell. 1993 Feb 26;72(4):635-48. doi: 10.1016/0092-8674(93)90081-z. PMID: 7679955.
- Wong P, Rudensky AY. Phenotype and function of CD4+ T cells in mice lacking invariant chain. J Immunol. 1996 Mar 15;156(6):2133-42. PMID: 8690902.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in