Ovulation in a dish: new insights into follicle rupture with implications for infertility and non-hormonal contraceptive development
Published in Protocols & Methods, Cell & Molecular Biology, and General & Internal Medicine
Ovulation is the most remarkable, autocatalytic, self-destructive event in all of biology through which half of our genome must emerge. Despite its necessity in reproduction, we are limited in what we know about ovulation, at least in part because the study of a structure that is programed for partial destruction is hard. Moreover, the idea that the surface of an internal organ is willingly destroying half of its structure and simultaneously transforming the other half into a necessary new structure is daft. But biology built this system and here, for the first time, we can study it. In this paper we unravel the molecular programming of ovulation, pointing the way toward better understanding of the process and creating new pathways to engineer fertility, build contraceptives, and identify mechanisms when ovulation fails.
At the time of ovulation, the egg is released from the side of the follicle wall facing the outside of the ovary (Figure 1). After ovulation, the remaining side of the follicle wall involutes to form a structure called the corpus luteum, which produces progesterone, a hormone needed to support early pregnancy. Over the past several decades, researchers have developed robust methods to grow follicles completely outside of the ovary (ex vivo) have been established.1-8 These methods recapitulate key steps in follicle development including ovulation (Video 1). Through these studies, we and other researchers have made the striking observation that follicles can be induced to undergo ovulation ex vivo, and follicle rupture occurs asymmetrically even in the absence of cues from the ovarian microenvironment (Figure 1).6 In fact, morphological features of follicles grown ex vivo can be used to predict the side where follicle rupture will occur even prior to ovulation induction. This observation suggests that the follicle wall contains intrinsic molecular programs that dictate which side will rupture (the ruptured side) and which will remain intact (the unruptured side).
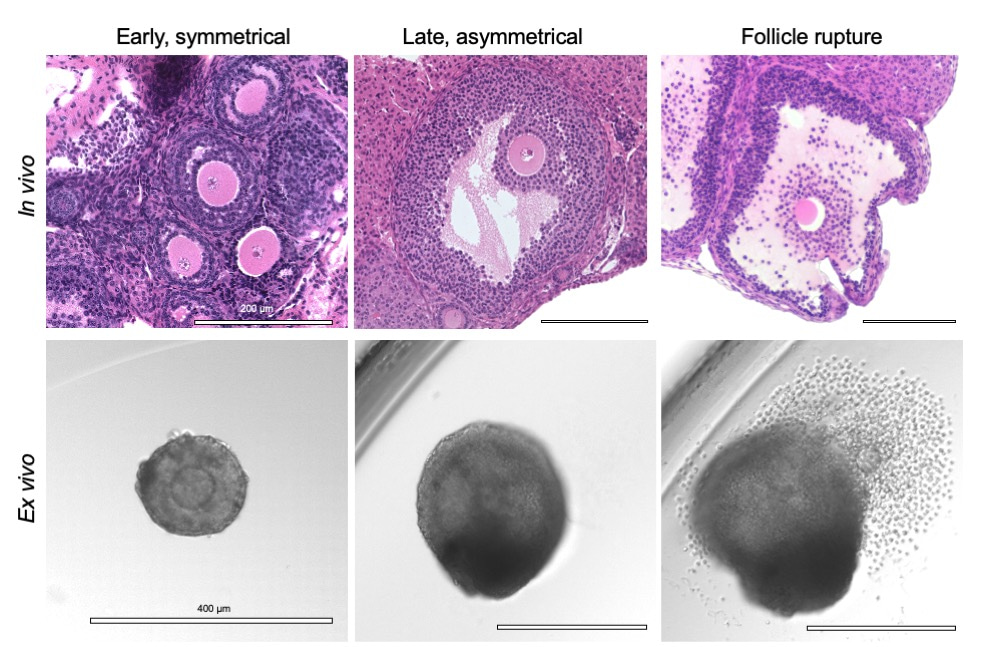
Figure 1. Ovulation is characterized by structural and molecular asymmetry. Ovarian follicles are the functional unit of the ovary, made up of somatic cells surrounding an oocyte. Follicles begin as symmetrical structures that grow and differentiate asymmetrically with formation of a fluid-filled antral cavity. This asymmetry peaks at the point of ovulation, where the follicle wall ruptures open to release a mature gamete. The asymmetry of follicle development and rupture is maintained in our ex vivo model despite removal of the follicle from the context of the ovary.
Our study uses this established ex vivo model to investigate what makes these regions of the follicle wall different. We discovered that after ovulation, the unruptured side of the follicle wall turns on machinery required for transformation into a corpus luteum, whereas the side that ruptures shows signs of cell death. Next, we trained a machine learning model to predict the location of follicle rupture within ex vivo grown follicles, and we used this model to mechanically separate the follicle sides for transcriptomic analysis using RNA sequencing. In our sequencing data, we identified distinct gene expression patterns that distinguished each side of the follicle wall. Our results confirmed known processes that occur asymmetrically during ovulation; for example, smooth muscle contraction and vascular changes on the unruptured side, and structural remodeling and inflammatory processes on the ruptured side.9-14 We also discovered several pathways that were previously not known to be involved in ovulation biology. On the unruptured side, we found enriched genes related to energy metabolism and unique signaling pathways (including Wnt, IL-11, and metal ion signaling). On the ruptured side, we found enrichment of genes related to the transport of amino acids as well as important molecular pathways (including bone morphogenenetic protein and Jag-Notch signaling).
Our findings have important implications beyond basic science. Impaired follicle rupture, which can result in trapped eggs that fail to be released from the ovary, may be a contributing factor in some cases of unexplained infertility among women. Notably, several genes—such as Insr, Adamts1, and Ptgs2—enriched in our dataset in the ruptured side of the follicle wall have been linked to infertility associated with trapped eggs in mouse loss-of-function models.15-17 Other genes highlighted in our dataset may also play crucial roles in follicle rupture and could shed light on causes of unexplained infertility. Additionally, our research has the potential to identify new targets for the development of non-hormonal contraceptives. The ability to inhibit follicle rupture without disrupting luteinization or the hormonal balance of the ovulatory cycle could represent a groundbreaking contraceptive strategy. Our lab is currently investigating which genes identified in our dataset could be targeted to create effective non-hormonal contraceptives.
In summary, our study uncovers novel, follicle-inherent, and spatially distinct processes that occur during follicle rupture. Further exploration of the genes identified in our analysis could significantly advance women’s health and reproductive biology.
References:
1 Simon, L. E., Kumar, T. R. & Duncan, F. E. In vitro ovarian follicle growth: a comprehensive analysis of key protocol variables†. Biology of Reproduction 103, 455-470 (2020). https://doi.org/10.1093/biolre/ioaa073
2 Zhang, J. et al. An ex vivo ovulation system enables the discovery of novel ovulatory pathways and nonhormonal contraceptive candidates†. Biology of Reproduction 108, 629-644 (2023). https://doi.org/10.1093/biolre/ioad009
3 Shea, L. D., Woodruff, T. K. & Shikanov, A. Bioengineering the Ovarian Follicle Microenvironment. Annual Review of Biomedical Engineering 16, 29-52 (2014). https://doi.org/https://doi.org/10.1146/annurev-bioeng-071813-105131
4 Parrish, E. M., Siletz, A., Xu, M., Woodruff, T. K. & Shea, L. D. Gene expression in mouse ovarian follicle development in vivo versus an ex vivo alginate culture system. Reproduction 142, 309 (2011).
5 Xu, M., Kreeger, P. K., Shea, L. D. & Woodruff, T. K. Tissue-engineered follicles produce live, fertile offspring. Tissue engineering 12, 2739-2746 (2006).
6 Converse, A., Zaniker, E. J., Amargant, F. & Duncan, F. E. Recapitulating folliculogenesis and oogenesis outside the body: encapsulated in vitro follicle growth. Biology of reproduction 108, 5-22 (2023).
7 Skory, R. M., Xu, Y., Shea, L. D. & Woodruff, T. K. Engineering the ovarian cycle using in vitro follicle culture. Human Reproduction 30, 1386-1395 (2015).
8 Xiao, S. et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 8, 14584 (2017). https://doi.org/10.1038/ncomms14584
9 Migone, F. F. et al. In vivo imaging reveals an essential role of vasoconstriction in rupture of the ovarian follicle at ovulation. Proceedings of the National Academy of Sciences 113, 2294-2299 (2016).
10 Ko, C. et al. Endothelin-2 in ovarian follicle rupture. Endocrinology 147, 1770-1779 (2006). https://doi.org/10.1210/en.2005-1228
11 Martin, G. G. & Talbot, P. Drugs that block smooth muscle contraction inhibit in vivo ovulation in hamsters. Journal of Experimental Zoology 216, 483-491 (1981).
12 Shkolnik, K. et al. Reactive oxygen species are indispensable in ovulation. Proceedings of the National Academy of Sciences 108, 1462-1467 (2011).
13 Emery, A. et al. Dynamic regulation of semaphorin 7A and adhesion receptors in ovarian follicle remodeling and ovulation. Frontiers in Cell and Developmental Biology 11 (2023).
14 Zaniker, E. J., Babayev, E. & Duncan, F. E. Common mechanisms of physiological and pathological rupture events in biology: novel insights into mammalian ovulation and beyond. Biological Reviews 98, 1648-1667 (2023). https://doi.org/https://doi.org/10.1111/brv.12970
15 Richards, J. S., Liu, Z. & Shimada, M. in Knobil and Neill's Physiology of Reproduction (Fourth Edition) (eds Tony M. Plant & Anthony J. Zeleznik) 997-1021 (Academic Press, 2015).
16 Robker, R. et al. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proceedings of the National Academy of Sciences 97, 4689-4694 (2000).
17 Duffy, D. M. Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Human Reproduction Update 21, 652-670 (2015). https://doi.org/10.1093/humupd/dmv026
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in