A joint diffusion/collision model for crystal growth in pure liquid metals
Published in Materials
The major finding of this work is that a considerable fraction of liquid atoms at the liquid/solid interface need thermal activation for growth to take place while the others attach to the crystal without any energy barrier. We reveal that the thermal activation of some liquid atoms at the growing interface is a general phenomenon, contradicted to previous belief of confusion theory that crystal growth of pure metals does not need thermal activation.
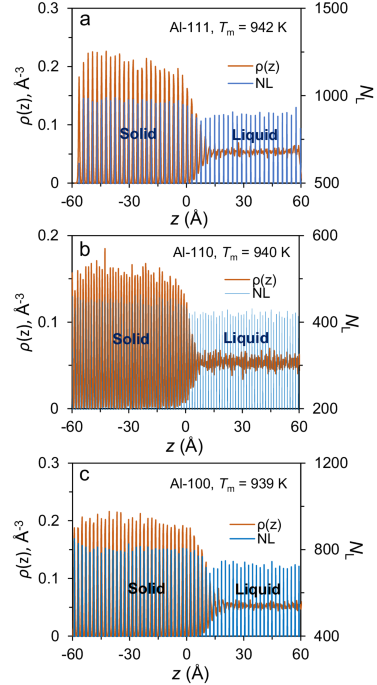
Although some doubts have been cast to the confusion theory in recent years and many high-quality studies have been reported [1-7], these studies always indicate that the thermal activation may need only in some specific cases. For example, Broughton et al. [8] revealed the concerted displacements at an FCC(111) interface, but this worked only for FCC(111) interface, not for other interfaces or BCC metals. On the other hand, Zhang et al [9] obtained nanometer-size metallic glasses of pure BCC metals with high liquid-quench technique, and demonstrated that the growth of pure BCC metals was diffusion-controlled using MD simulations. Recently, Zhenzhen Yan et al [7,10] and Gang Sun et al. [5] confirmed the diffusion-controlled growth for BCC metals while the same studies argued that the growth of FCC metals was an almost barrier-less process [7] or with little to no activation energy [5]. In literature, many studies are purposefully performed to explain a single experimental result, e.g., metallic glasses with size of up to 100 nm can be obtained for BCC metals with super cooling rate, but not for FCC metals, and ignore the general behaviour of growth kinetics for simple materials (this seems true for a lot of simulation works, i.e., they can prove something is correct or wrong if it supposed to be that with their modelling and mathematics skills, but it is not necessary the real nature of the problems! Surely, I don’t underestimate the contributions and significance of these works). It is noted that it is still very difficult to produce the metallic glasses of BCC pure metals, with nanometre size and constrained geometrical condition [9], and the diffusion-controlled mechanism for BCC metals is not consistent with previous experimental observation [11-13], as showed below. In general, pure BCC and FCC metals (as simple materials) exhibit very similar behaviours of crystal growth: (1) very high growth velocity up to 100 m/s from experimental measurements, which is too large to be the result of diffusion-controlled process and in favour of collision theory (such as for BCC Fe, Ti; HCP Zr; FCC Co, Al, Au, Cu, Ni [11-13]); (2) growth velocity reaches the maximum at about 0.7 Tm (so-called Tc, crossover or turnover temperature) [4], which can’t be interpreted by collision theory alone due to the weak temperature dependence; (3) crystallization of pure metals at a temperature as low as 4 K [14], which can’t be interpreted by diffusion theory alone where diffusion rate literally drops to zero. Further, the growth kinetics behaviour of these simple materials (include pure BCC metals) is significantly different from that of the complex materials, for which it has been well established that the growth is diffusion-controlled and usually the crossover of growth velocity can be observed at a high temperature of 0.9-0.95 Tm [15,16]. All these indicates that the growth kinetics of pure metals is not governed by a single mechanism, either collision or diffusion theory.
In this very active research field of the growth interface kinetics, as indicated by Refs. [1-7], there are many confused, biased and flawed answers to the long-standing fundamental question in crystal growth: whether or not the crystal growth of pure metals is thermal activated. Our study provides a decisive solution for this question: the growth of pure metals is a mixed process of collision-limited and diffusion-controlled process. Our joint collision/diffusion model developed in this study can explain these phenomena reasonably with a single fitting parameter of the fraction of liquid atoms with thermal activation at the interface: (1) a majority of liquid atoms at the interface don’t need thermal activation, and so a high growth velocity can be achieved; (2) a fraction of liquid atoms need thermal activation, leading to the crossover of growth velocity; (3) crystallization of pure metals can occur at high undercooling due to the partial collision mode; (4) the crossover temperature is about 0.7-0.8 Tm for pure metals, lower than 0.9-0.95 Tm for complex materials, since only a fraction of liquid atoms for simple metals is thermal activated and makes a smaller contribution to the slowing interface kinetics than complex materials. It suggests that our study reveals the genuine mechanism of growth kinetics for simple materials, and the reasonably good fitting with our analytical model to the MD simulation data gives further validation for the mechanism of interface kinetics proposed in the present study.
This study represents a significant leap over the current state of the very active research field in interface kinetics by ending the confusion over the role of collision-limited and diffusion-controlled modes in crystal growth. Our finding in this study is a significant advancing and breakthrough in the solidification theory by providing a profound understanding on mechanism of the interface kinetics, as well as our joint collision/diffusion model to calculate the growth kinetics reasonably well, which is one of key parameters used in modelling of solidification and phase change materials.
References:
[1] Hu, Y. -C. & Tanaka, H. Revealing the role of liquid preordering in crystallisation of supercooled liquids. Nat. Commun. 13, 4519 (2022).
[2] Freitas, R. & Reed, E. J. Uncovering the effects of interface-induced ordering of liquid on crystal growth using machine learning. Nat. Commun. 11, 3260 (2020)
[3] Sun, G., Xu, J. & Harrowell, P. The mechanism of the ultrafast crystal growth of pure metals from their melts. Nat. Mater. 17, 881 (2018)
[4] Ashkenazy, Y. & Averback, R. S. Kinetic stages in the crystallization of deeply undercooled body-centered-cubic and face-centered-cubic metals. Acta Mater. 58, 524-530 (2010)
[5] Sun, G., Hawkena, A. & P. Harrowell, The displacement field associated with the freezing of a melt and its role in determining crystal growth kinetics, PNAS 117, 3421–3426 (2020)
[6] Schoenholz, S. S., Cubuk, E. D., Sussman, D. M., Kaxiras E. & Liu, A. J. A structural approach to relaxation in glassy liquids, Nature Physics, 12, 469 (2016)
[7] Yan, Z. Z., Sheng, H., Ma, E., Xu, B., Li, J. F. & L.T. Kong, Intermediate structural evolution preceding growing BCC crystal interface in deeply undercooled monatomic metallic liquids, Acta Mater. 202, 387–398 (2021).
[8] Broughton, J. Q., Gilmer, G. H. & Jackson, K. A. Crystallization rates of a Lennard-Jones liquid. Phys. Rev. Lett. 49, 1496-1500 (1982).
[9] Zhong, L., Wang, J. W., Sheng, H.W., Zhang, Z.& Mao, S. X. Formation of monatomic metallic glasses through ultrafast liquid quenching, Nature, 512, 177 (2014).
[10] Yan, Z. Z., Xu, B., Wang, F. F., Li, J. F. & Kong, L. T. Effects of undercooling on atomic crystallization behaviors and growth mechanisms of pure metals, J. Appl. Phys. 132, 075301 (2022)
[11] Coriell S. R. & Turnbull, D. Rapid growth of crystals in undercooled melts, Acta metall. 30, 2135 (1982).
[12] MacDonald, C. A., Malvezzi, A. M. & Spaepen, F. Picosecond time resolved measurements of crystallization in noble metals. J. Appl. Phys. 65, 129-136 (1989).
[13] Vitta, S. Rapid solidification of polymorphic transition metals induced by nanosecond laser pulses. Appl. Phys. Lett. 59, 411-413 (1991).
[14] W. Rühl & P. Hilsch, Superconductivity and normal electrical conductivity of amorphous lead films nucleated with molybdenum, Z. Phys. B 26, 161 (1977)
[15] Ediger, M. D., Harrowell, P. & Yu, L. Crystal growth kinetics exhibit a fragility-dependent decoupling from viscosity. J. Chem. Phys. 128, 034709 (2008).
[16] Powell, C. T., Paeng, K., Chen, Z., Richert, R., Yu, L. & Ediger, M. D. Fast crystal growth from organic glasses: Comparison of o‑Terphenyl with its structural analogs. J. Phys. Chem. B 118, 8203−8209 (2014).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in