A New Electrolyte for Molten Carbonate Decarbonization" - Transformative Research for CO2 Removal
"A New Electrolyte for Molten Carbonate Decarbonization," an advancement in decarbonization technology targeting the urgent need for large-scale carbon dioxide removal to combat climate change. Reshape how we approach carbon sequestration and graphene nanocarbon (GNC) production.
Published in Earth & Environment, Materials, and Education
In the recently published article, *"A New Electrolyte for Molten Carbonate Decarbonization,"* by Gad Licht, Kyle Hofstetter, Xirui Wang, and Stuart Licht, a groundbreaking advancement in decarbonization technology is introduced, targeting the urgent need for large-scale carbon dioxide (CO2) removal to combat climate change. Published in *Communications Chemistry* (Nature) in 2024, this paper dives into innovative chemistry that could reshape how we approach carbon sequestration and graphene nanocarbon (GNC) production.Overview of the Study
The study explores a novel method for transforming CO2 into oxygen and valuable carbon nanomaterials, such as carbon nanotubes (CNTs), through a molten carbonate electrolysis process. Previous efforts in this field relied heavily on lithium carbonate (Li₂CO₃), which has seen its demand surge due to its use in electric vehicle (EV) batteries, making it an expensive and limited resource. The authors propose a more cost-effective and abundant alternative in the form of strontium carbonate (SrCO₃), presenting a path to sustainable carbon removal.
Background: The C2CNT Process
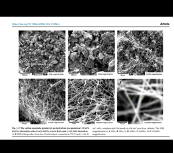
The research builds on a 2015 innovation, the C2CNT (carbon dioxide to carbon nanomaterial technology) process, which transforms CO2 into solid carbon allotropes such as CNTs via electrolysis in molten carbonates. The carbon nanotubes produced are highly valued for their strength, conductivity, and potential applications in industries ranging from electronics to medicine. The challenge, however, lies in the cost and limited availability of Li₂CO₃, which has made scaling up this technology difficult. In this new study, Licht and colleagues focus on using SrCO₃, an abundant and affordable alternative to Li₂CO₃, to make the C2CNT process more viable on a large scale.
Key Innovation: Strontium Carbonate as a Replacement
Strontium carbonate is not only cheaper and more abundant than lithium carbonate, but it also has comparable properties, not retained by the other carbonates, in terms of its ability to absorb and release CO2. The study demonstrates that SrCO₃ can be used in conjunction with a small percentage of Li₂CO₃ (30% or less) to produce CNTs at a lower cost, while sequestering CO2 in a stable and useful form. This is a significant finding as it reduces reliance on the costly Li₂CO₃, while maintaining the high purity of CNTs. The net result is a unique, scalable decarbonization process that produces revenue while at the same time removing the greenhouse gas.
The authors also note that SrCO₃'s solidus temperature is 1494°C, much higher than that of Li₂CO₃. However, despite its high melting point, SrCO₃ is surprisingly soluble in Li₂CO₃ at temperatures under 800°C, allowing it to be effectively used in the electrolysis process. This discovery opens up new possibilities for large-scale carbon sequestration using SrCO₃-based electrolytes.
Comparative Analysis: Li₂CO₃ vs. SrCO₃
The study offers a detailed comparison between the performance of Li₂CO₃ and SrCO₃ in molten carbonate electrolysis. While Li₂CO₃ has been the standard for producing CNTs via the C2CNT process, its high cost—driven by competition from the EV battery market—has made it less practical for large-scale decarbonization efforts. SrCO₃, on the other hand, is an orders of magnitude cheaper and more readily available. The authors emphasize that the electrochemical properties of SrCO₃, specifically its affinity for CO2, are nearly identical to those of Li₂CO₃, making it a viable substitute.
Environmental and Economic Impact
One of the most compelling aspects of this research is the potential environmental and economic impact of switching to SrCO₃-based electrolytes. The production of CNTs from CO₂ not only helps remove greenhouse gases from the atmosphere but also creates a valuable product with wide-ranging applications. CNTs are used in the manufacturing of high-strength materials, electronic components, and medical devices, among other things. The current market value of CNTs is approximately $1 million per ton, providing a significant incentive for carbon removal.
The cost analysis provided in the article highlights the stark difference between using Li₂CO₃ and SrCO₃. With Li₂CO₃ priced between $10,000 and $75,000 per ton, the cost of producing CNTs via the C2CNT process can be prohibitive. In contrast, SrCO₃ costs around $1,040 per ton, making it a far more economical choice for large-scale decarbonization efforts.
Challenges and Future Research
The authors call for further exploration of the practical applications of CNTs produced through this method. The high strength and conductivity of CNTs make them ideal for use in a variety of industries, and their production from CO2 offers a sustainable solution to two pressing global problems: carbon emissions and the demand for advanced materials.
The research presented in *"A New Electrolyte for Molten Carbonate Decarbonization"* is a significant step forward in the quest for sustainable carbon removal. By demonstrating the feasibility of using SrCO₃ as a cost-effective and abundant alternative to Li₂CO₃, the authors provide a pathway for scaling up the C2CNT process to remove CO2 from the atmosphere while producing valuable carbon nanomaterials. As climate change continues to pose an existential threat to the planet, innovations like this offer hope for a future where we can not only mitigate the effects of carbon emissions but also create valuable products in the process.
For more information and to delve into the details of this groundbreaking research, the full article can be accessed through *Communications Chemistry*: https://www.nature.com/articles/s42004-024-01306-z.epdf?sharing_token=7SJ-FG2E3g3r_bXacMHsFtRgN0jAjWel9jnR3ZoTv0Mteaq3IEYP_rGkt6tosyL5wcAIt380cy_VDikKWdC5ifmXr167LEc0ctuvWX4Tn3p7zgNbwAZ7VuVkylX6jEsnJqjncddFzoJlhbHzKZ1MHVX_WJ3uiERxk_5aqJKvqJE%3D
or contacting https://carboncorp.org/contact.
Follow the Topic
Environmental and Sustainability Education
Humanities and Social Sciences > Education > Environmental and Sustainability Education
Materials Chemistry
Physical Sciences > Chemistry > Materials Chemistry
Climate Sciences
Physical Sciences > Earth and Environmental Sciences > Earth Sciences > Climate Sciences
Carbon Materials
Physical Sciences > Chemistry > Materials Chemistry > Carbon Materials
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
This Collection aims to highlight recent progress in f-element chemistry, encompassing studies on fundamental electronic structure, advances in separation chemistry, advances in coordination and organometallic chemistry, and the application of f-element compounds in materials science and environmental technologies.
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
This cross-journal Collection highlights methodological developments in instrument design, sample preparation, data acquisition, data analysis, interpretation and integration from different techniques.
Publishing Model: Open Access
Deadline: Apr 30, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in