A SYS1–ARFRP1–ARL5–ARMH3–PI4KB axis regulates PI4P synthesis at the trans-Golgi network
Published in Cell & Molecular Biology
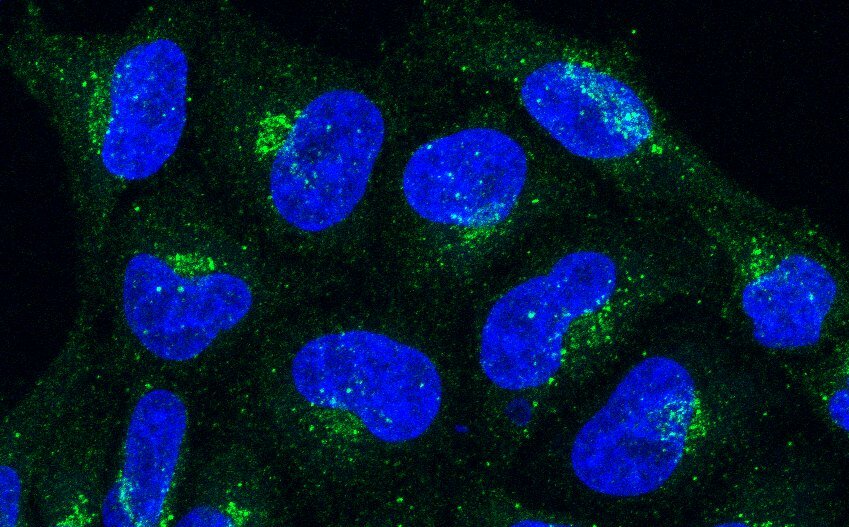
Phosphatidylinositol 4-phosphate (PI4P) is an anionic phospholipid that is highly enriched in the membranes of the Golgi complex. Through interactions with PI4P-binding proteins, Golgi PI4P regulates diverse processes, including exocytic cargo export, glycan modifications, membrane contacts with the endoplasmic reticulum (ER), non-vesicular lipid transport, sphingomyelin synthesis, STING activation, and viral replication1. Humans have four phosphatidylinositol 4-phosphate kinases, PI4KA, PI4KB, PI4K2A and PI4K2B, with PI4KB being the primary enzyme responsible for PI4P synthesis at the Golgi complex. However, the mechanisms regulating this synthesis remained incompletely understood. In a recent study2, we demonstrate that the majority of Golgi PI4P synthesis is controlled by a SYS1–ARFRP1–ARL5–ARMH3–PI4KB axis. SYS1 is a multispanning membrane protein that anchors the small GTPase ARFRP1 to the trans-Golgi network (TGN), the most distal compartment of the Golgi complex. The SYS1-ARFRP1 complex then promotes the recruitment of another small GTPase, ARL5, to the TGN3. ARL5 subsequently recruits its effector ARMH3 (also known as C10orf76), which in turn activates PI4KB at the TGN2. Disruption of this axis reduces Golgi PI4P levels, impairing the recruitment of the PI4P-binding adaptor protein GOLPH3 and causing partial defects in glycan modifications on a subset of glycoproteins2. The components of the SYS1–ARFRP1–ARL5–ARMH3–PI4KB axis are highly conserved among eukaryotes4,5, suggesting a fundamental role of this axis in Golgi PI4P regulation.
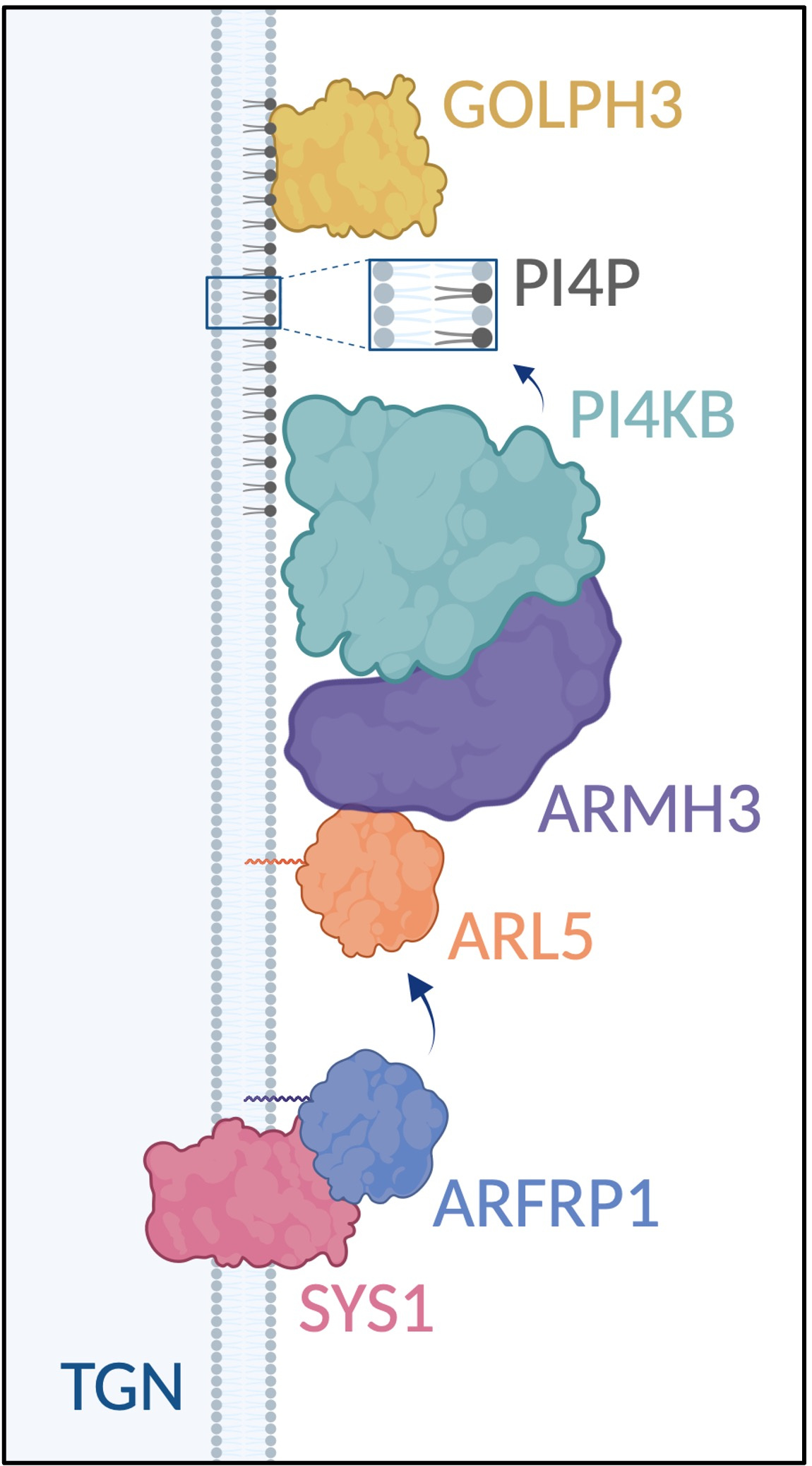
SYS1–ARFRP1–ARL5–ARMH3–PI4KB axis regulates PI4P synthesis at the trans-Golgi network (TGN). Schematic created in BioRender. Bonifacino, J. (2024) BioRender.com/o49h844.
This work was initiated by Dr. Tal Karen-Kaplan in our laboratory at the NICHD, aiming to identify effector proteins of ARF-like (ARL) small GTPases using the recently developed MitoID technique6. Unlike classical proximity labeling methods, MitoID relocates bait proteins to mitochondria, improving the detection of bona fide soluble interactors while minimizing labeling of non-interacting neighbors at the ARLs’ original location. The MitoID experiments began in March 2020, just before the extended quarantine period caused by the COVID-19 pandemic. In anticipation of our laboratory’s shutdown, we rushed to collect and freeze the samples. After several months, we were able to return on a rotational basis and submit the samples to Dr. Yan Li for mass spectrometry analysis. Fortunately, the samples withstood the prolonged freezing, ultimately leading to the identification of ARMH3 as an ARL5 effector. In January 2022, we began to functionally characterize the interaction between ARL5 and ARMH3, and their partner PI4KB.
Luckily, our neighbors down the corridor, Dr. Joshua Pemberton and Dr. Tamas Balla, also at the NICHD, were involved in a previous study that identified the ARMH3-PI4KB complex as important for generating PI4P at the Golgi complex and for ARMH3-dependent enterovirus replication7. In addition to advising us on study design, they also forewarned us about the technical challenges of studying ARMH3. Indeed, one of the most difficult aspects of this study was constructing and propagating plasmids containing the complete ARMH3 coding sequence. Although we could easily amplify full-length ARMH3 from HeLa cDNA by PCR, ARMH3-positive clones barely survived after transformation of the ligation products, and almost all surviving clones contained frameshift mutations. This suggested that full-length ARMH3 was toxic to the host, most likely due to leaky expression driven by the plasmids' viral promoter8. After reading blog posts by scientists facing similar issues on ResearchGate, we modified the protocol by changing the strain of E. coli and lowering the culture temperature to 20°C. Eventually, we succeeded in getting plasmids encoding full-length ARMH3, made glycerol stocks of sequence-confirmed clones for future plasmid preparation, and continued to sequence-verify every new preparation before the experiments.
Using these plasmids, we obtained strong evidence for interactions between ARL5, ARMH3, and PI4KB in cell-based assays, including mitochondrial relocalization and yeast two- and three-hybrid assays. However, we struggled to confirm these interactions using in vitro biochemical methods. A co-immunoprecipitation assay using HEK293T cells expressing ARL5 and ARMH3 detected only a weak interaction, which contrasted sharply with the strong interaction observed in the in vivo assays. Additionally, a GST-pulldown assay with GST-ARL5 constructs failed to show any interaction with ARMH3, even when the non-hydrolyzable GTP analog GTPγS was added to the reaction.
Fatefully, one of us (Dr. Morié Ishida) met Dr. Rahul Raina, an NICHD structural biologist studying GTPases, at a baby shower hosted by another lab member. Dr. Ishida shared his struggles with the binding experiments, and Dr. Raina kindly suggested trying a potassium-based buffer that had worked well in his own research. Back in the lab, we swapped our standard sodium-based lysis buffer for the potassium-based one, and the binding results were dramatically improved—visible even by gel staining, and without GTPγS! This is in line with reports suggesting that potassium acts as a GTPase-activating agent9,10, which may also apply to ARL5. This protocol adjustment significantly strengthened our evidence for these interactions and served as a prime example of how open scientific discussions can be beneficial to everyone.
Thus, despite the challenges, we ultimately reached a satisfactory conclusion to our project. We identified ARMH3 as a novel effector of ARL5 and demonstrated that the SYS1–ARFRP1–ARL5–ARMH3–PI4KB axis is essential for maintaining the majority of the PI4P pool at the Golgi complex. These findings integrate and expand upon previous studies on ARL5 and ARMH3 in Golgi PI4P activation and recruitment7,11,12. Our research also underscores the diverse roles of ARL5 at the TGN, including its established function in promoting SNARE-dependent fusion of endosome-derived carriers to the TGN through a previously known effector, the GARP complex, as well as its newly identified role in regulating PI4P synthesis and downstream processes at the TGN via ARMH3.
Many questions remain to be addressed, including: Do ARL5, ARMH3, and PI4KB form a tripartite complex and, if so, what is its structure? How do ARL5 and ARMH3 cooperate to activate PI4KB? What specific roles do other interactors of ARMH3 and PI4KB, such as GBF1 and ACBD3, respectively, play in PI4KB regulation? Does ARL5 have additional effectors, and if so, which TGN processes do they regulate? Answering these questions will further elucidate the mechanisms underlying ARL5 function at the TGN and provide deeper insights into its role in cellular processes.
References
- De Matteis, M. A., Wilson, C. & D’Angelo, G. Phosphatidylinositol-4-phosphate: the Golgi and beyond. Bioessays35, 612-622 (2013).
- Ishida, M. et al. ARMH3 is an ARL5 effector that promotes PI4KB-catalyzed PI4P synthesis at the trans-Golgi network. Nat Commun 15, 10168 (2024).
- Ishida, M. & Bonifacino, J. S. ARFRP1 functions upstream of ARL1 and ARL5 to coordinate recruitment of distinct tethering factors to the trans-Golgi network. J Cell Biol 218, 3681-3696 (2019).
- Rosa-Ferreira, C., Christis, C., Torres, I. L. & Munro, S. The small G protein Arl5 contributes to endosome-to-Golgi traffic by aiding the recruitment of the GARP complex to the Golgi. Biol Open 4, 474-481 (2015).
- Chan, C. J. et al. BioID Performed on Golgi Enriched Fractions Identify C10orf76 as a GBF1 Binding Protein Essential for Golgi Maintenance and Secretion. Mol Cell Proteomics 18, 2285-2297 (2019).
- Gillingham, A. K., Bertram, J., Begum, F. & Munro, S. In vivo identification of GTPase interactors by mitochondrial relocalization and proximity biotinylation. Elife 8, e45916 (2019).
- McPhail, J. A. et al. Characterization of the c10orf76-PI4KB complex and its necessity for Golgi PI4P levels and enterovirus replication. EMBO Rep 21, e48441 (2020).
- Lewin, A., Mayer, M., Chusainow, J., Jacob, D. & Appel, B. Viral promoters can initiate expression of toxin genes introduced into Escherichia coli. BMC Biotechnol 5, 19 (2005).
- Scrima, A. & Wittinghofer, A. Dimerisation-dependent GTPase reaction of MnmE: how potassium acts as GTPase-activating element. EMBO J 25, 2940-2951 (2006).
- Ash, M.-R. et al. Potassium-activated GTPase reaction in the G Protein-coupled ferrous iron transporter B. J Biol Chem 285, 14594-14602 (2010).
- Li, F. L. et al. Defining the proximal interaction networks of Arf GTPases reveals a mechanism for the regulation of PLD1 and PI4KB. EMBO J 41, e110698 (2022).
- Mizuike, A., Sakai, S., Katoh, K., Yamaji, T. & Hanada, K. The C10orf76-PI4KB axis orchestrates CERT-mediated ceramide trafficking to the distal Golgi. J Cell Biol 222, e202111069 (2023)
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in