An Unorthodox Glycosyltransferase Activity of Cellulose Synthase-Like Enzymes as an Example of Evolutionary 'Hijacking'
Published in Chemistry

I am not a storyteller, but I like to ask questions and find answers. That is why, this Behind the Paper post will be in form of Q&As.
What is so special about saponins?
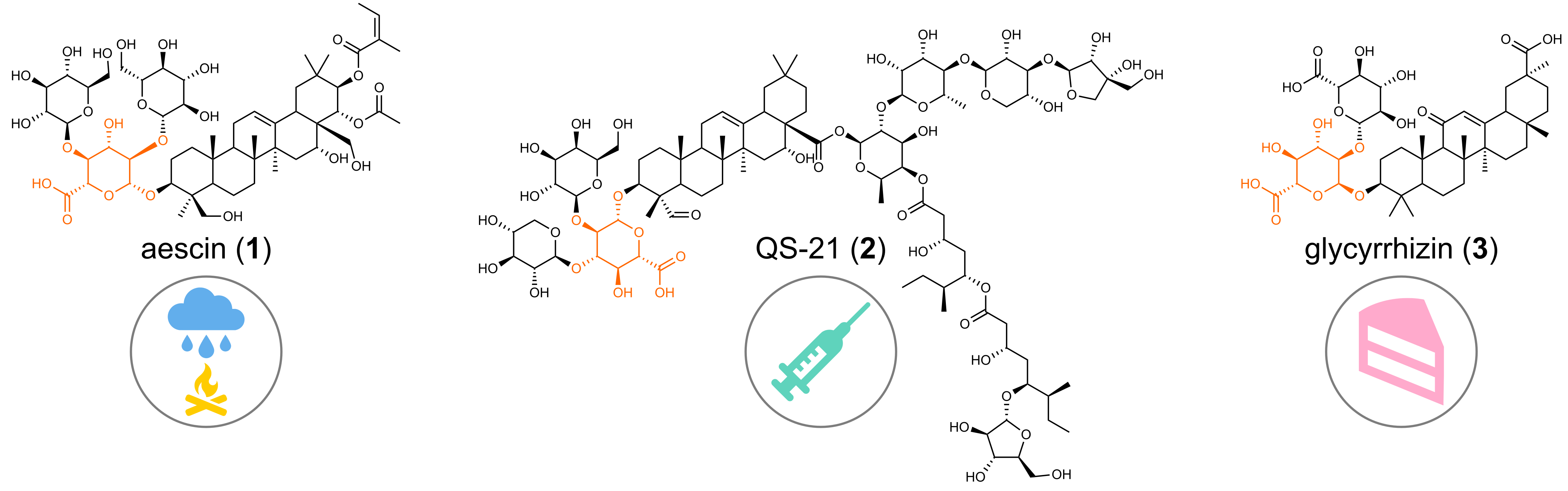
Saponins are a large group of specialized metabolites with soap like properties found in countless plant species. The unique physicochemical properties of these compounds provide a broad spectrum of functions in plants including antifungal and insecticidal activities. Plentiful reports underlined their benefits to human health and medical applications starting from anti-inflammatory (aescin (1) from horse chestnut) and anti-cancer properties to utilization as vaccine adjuvants (QS-21 (2) from soapbark tree). Triterpenoid saponins are also exploited in the food industries, e.g., glycyrrhizin (3) isolated from licorice is used as a low-calorie sweetener.
How do we get reasonable amounts of bioactive saponins?
Commercial production of aescin, glycyrrhizin and QS-21 depends on the inefficient industrial processing and the limited availability of naturally grown licorice and soapbark leading to significant environmental issues. Despite its importance, sustainable production of these potent saponins in heterologous systems is currently impossible due to lack of fully characterized biosynthetic pathways and especially unidentified enzymes catalyzing the attachment of glucuronic acid (GlcA) to the aglycone.
Why spinach?
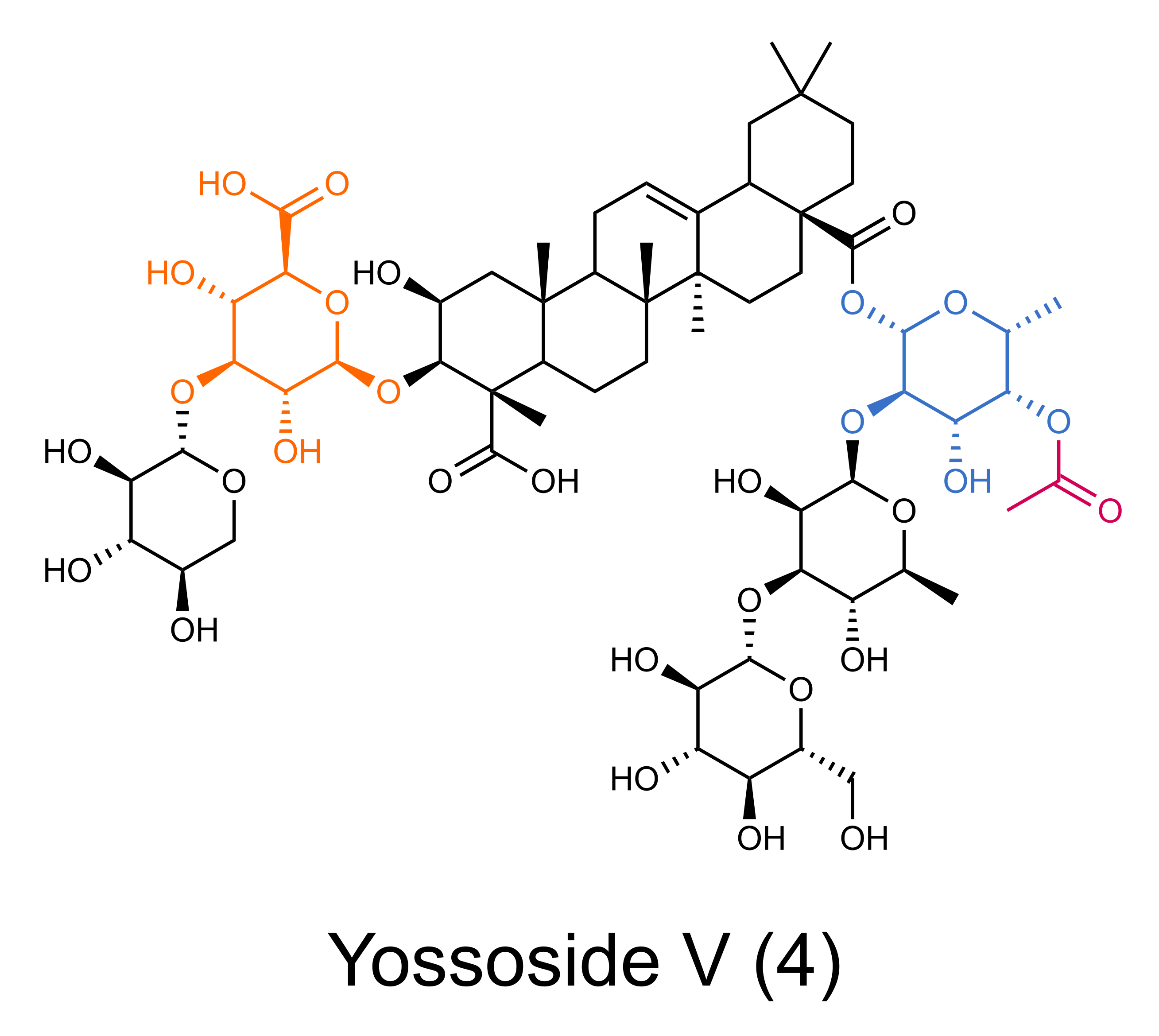
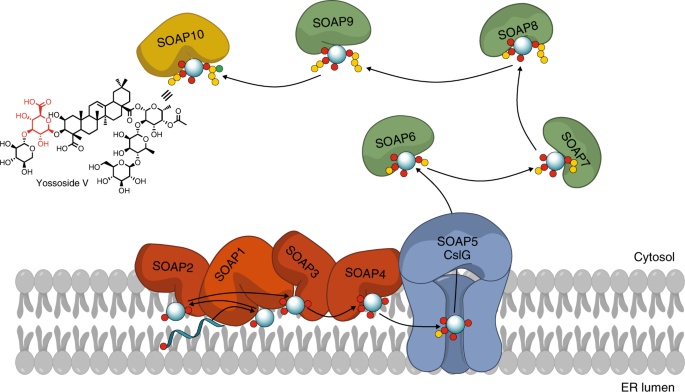
This question has been asked numerous times by many researchers, colleagues and friends. It especially reminds me of a TERPNET meeting in Dalian, China (2017) where I was presenting a poster with preliminary results and one of the most knowledgeable saponin researchers, professor Toshiya Muranaka asked me ‘Why spinach?’. Spinach is a member of the order Caryophyllales that includes many (edible) plants producing glucuronide type triterpenoid saponins with unknown biosynthetic pathways. It is fast growing diploid plant with the sequenced genome. In our metabolic screening survey, we have found that spinach produces high quantities of triterpenoid saponins. The most abundant one, Yossoside V (4), comprises of the aglycone (medicagenic acid) decorated with glucuronic acid (in orange in 4) at position C-3, acetyl-fucose (pink and blue) at C-28 and other three sugars. Structure similarity to aescin, glycyrrhizin and especially to QS-21 encouraged us to decipher its biosynthetic pathway.
How did we characterize the entire pathway?
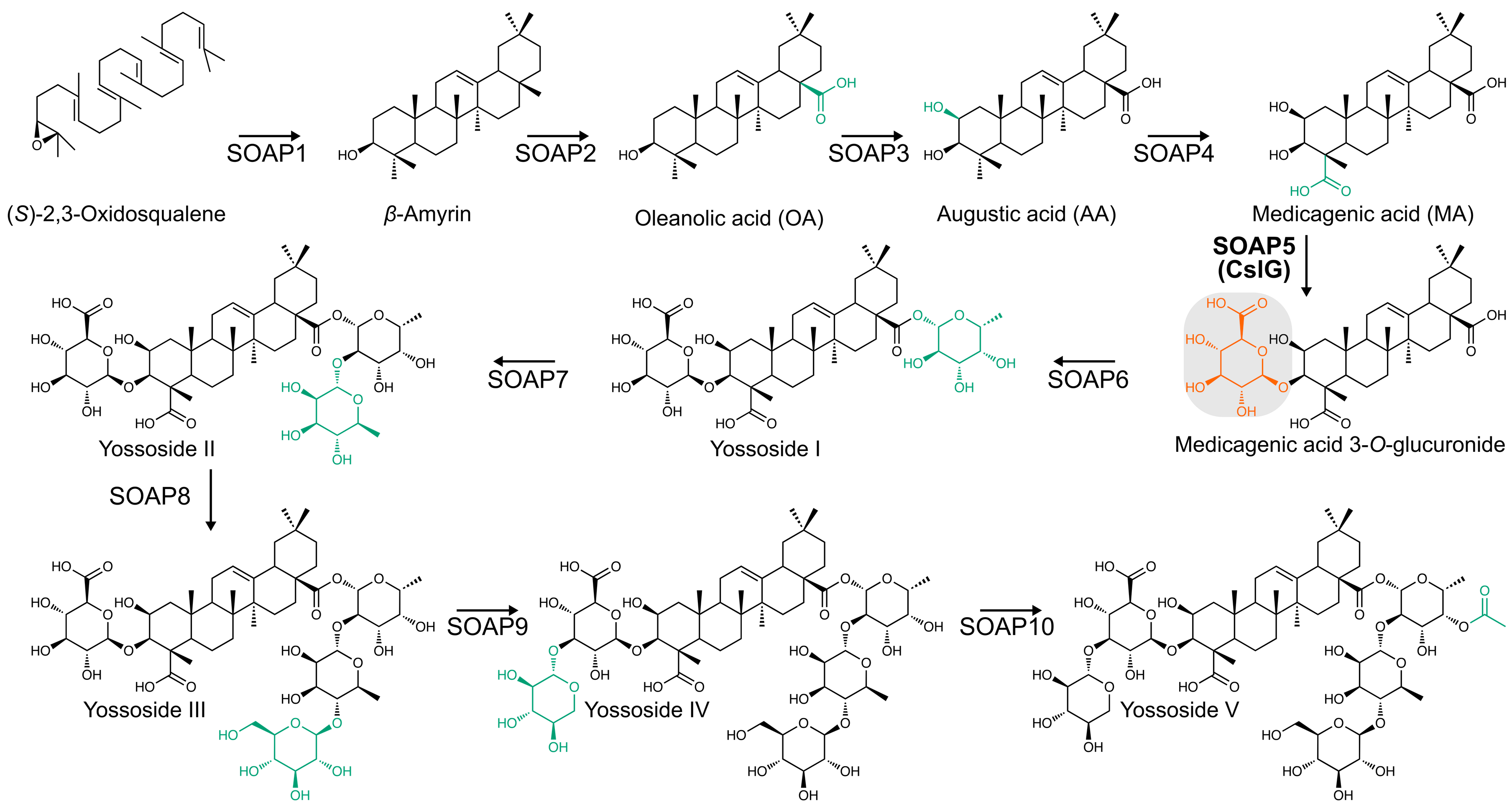
The process was relatively straightforward apart from one step. We have generated transcriptome data for five tissues with varying amount of saponins and used spinach orthologues of β-amyrin synthase (bAS, SOAP1) and cytochrome CYP716A268 (SOAP2) as baits in coexpression analysis that resulted in a list of candidate genes. We transiently expressed all the coexpressed genes that could be involved in the production of Yossosides. Yet, in all these experiments we could produce only the aglycone. The only gene coexpressed with all the baits in the coexpression set that appeared to be related to sugar metabolism was a spinach homolog of Cellulose synthase like G (SoCslG). Although its contribution to saponin biosynthesis seemed unlikely based on its annotation, we proceeded to study its function. I will never forget my PI (Asaph Aharoni) saying: “try to use the cellulose synthase you have found”. This led us to the discovery of first glucuronic acid transferase acting on triterpenoid aglycones.
Is that true only for spinach?
No, it is a more common phenomena and CslG enzymes attach GlcA to triterpenoid aglycones also in other plant species. Phylogenetic analysis of csl proteins allowed us to find functional orthologues of SOAP5 in other species from the Caryophyllales and Fabales orders. Discovery of CslG enzymes in licorice and soybean completed missing steps in biosynthetic pathways of glycyrrhizin and soyasaponins.
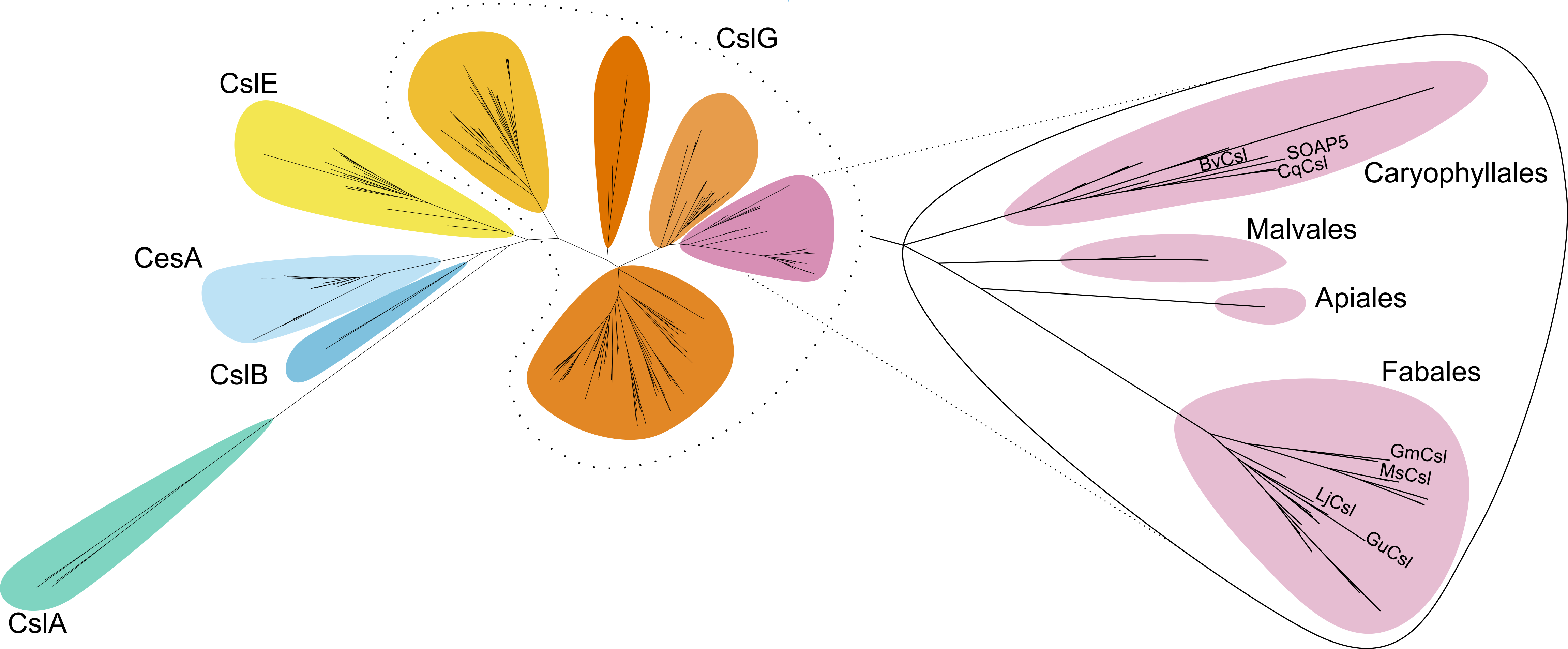
It seems weird that cellulose synthase like enzyme plays role in saponin production, right?
The unexpected finding of CslG proteins activity as triterpenoid glucuronosyltransferases demonstrates that glycosylation of specialized metabolites is not exclusively executed by family 1 type UGTs. Moreover, it provides a fundamental example of how enzyme activity in one of the few principal plant processes, i.e., the cellulose synthesis machinery, is ‘hijacked’ in order to produce a set of unrelated, defense dedicated metabolites. Colocalization of SOAP5 at the spinach ER membrane with most other pathway proteins rather than the cytosol (in which UGTs localize) was part of evolving its new function. It suggests the importance of physical proximity between these enzymes for efficient triterpenoids production.
Is there something unique about the other findings?
Apart from characterizing SOAP5 as glucoronic acid transferase, we have identified the first fucosyltransferase acting on specialized metabolites (i.e. SOAP6) as all plant fucosyltransferases to date were associated with fucosylation of cell wall polysaccharides and proteins. We have also unveiled a BAHD-type acyltransferase (SOAP10) acetylating the C-28 fucose moiety of spinach triterpenoid saponins. With similarity to glucuronidation, the acylated fucose domain in triterpenoid saponins is important for their efficacy as therapeutic agents, e.g., adjuvanticity of the renowned QS-21, a potent saponin vaccine adjuvant from soapbark tree.
How does the world benefit from our research?
Metabolic engineering of the entire saponin biosynthetic pathways could be used in crop improvement. Furthermore, advanced synthetic biology and microbial fermentation of saponins will enable profound studies of their bioactivity and large-scale production of high-value products independent of cultivation or harvest in nature. In summary, this work provides a springboard for unlocking a substantially uncharted chemical space of potent saponins with enormous potential in diverse industries and agriculture.
Need more answers?
Check out our paper here: https://www.nature.com/articles/s41589-020-0541-x
Or visit our website: http://www.weizmann.ac.il/plants/aharoni/
Follow the Topic
-
Nature Chemical Biology

An international monthly journal that provides a high-visibility forum for the chemical biology community, combining the scientific ideas and approaches of chemistry, biology and allied disciplines to understand and manipulate biological systems with molecular precision.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in