Association between trajectories of adherence to endocrine therapy and risk of treated breast cancer recurrence among US nonmetastatic breast cancer survivors
Published in General & Internal Medicine
Breast cancer is the most common cancer diagnosed in the United States (US) and millions of women are affected every year.1 Prescription endocrine medication therapy is the mainstay treatment to reduce risk of a recurrence of breast cancer in survivors.2,3 During the first year of endocrine therapy use, nearly 30% of breast cancer survivors are nonadherent,4 which may increase breast cancer recurrence risk.
Our team wondered if someone had found patterns of adherence to endocrine therapy that were associated with greater risk of breast cancer recurrence. After looking it up, we saw that other researchers had found conflicting results regarding the association of endocrine therapy adherence and the risk of breast cancer recurrence.5-11 Also, none had identified the extent or patterns of adherence to endocrine therapy that were most associated with the risk of breast cancer recurrence across different cancer stages.
We wondered if we could find the patterns of endocrine therapy adherence that were most associated with the risk of breast cancer recurrence. We used the 2010-2019 SEER Medicare data set, which covers 35% of the US cancer population and includes 97% of individuals aged 65 and older in the US.12-14 We then narrowed down our study population to women who were US residents with Stages 0 to 3 breast cancer who had started at least 1 endocrine therapy prescription within 1 year of receiving a breast cancer diagnosis. We separated the women into two groups based on their breast cancer stages (Group 1=Stages 0-1; Group 2=Stages 2-3), because the risk of recurrence increases with the stage. After we had our study population, we created a model that measured their endocrine therapy use patterns within a year of initiation and we followed patients until the first treated breast cancer recurrence event, death, they switched to a Medicare Advantage plan or the end of the study period within 4 years after the women had finished taking one year of endocrine therapy. To do this we applied group-based trajectory models to identify distinct endocrine therapy adherence patterns.15-17
First, we found the same 5 patterns of adherence to endocrine therapy in the women with Stages 0-1 breast cancer as in the women with Stages 2-3 (Figure 1). Those patterns were (1) discontinuation before 6 months, (2) nonadherent after 6 months, (3) intermittent users, (4) consistently adherent with more than 80% of the proportion of days covered by a prescription fill, and (5) nearly perfect adherence. Then, we found that the women most associated with an increased risk of treated breast cancer recurrence compared to women with nearly perfect adherence were the women who discontinued endocrine therapy before 6 months. In the women with Stages 0-1 breast cancer, that risk was 84% higher and in the women with Stages 2-3 breast cancer, that risk was 38% higher. The women who discontinued endocrine therapy before 6 months after initiation were about 7% of the women in our study.
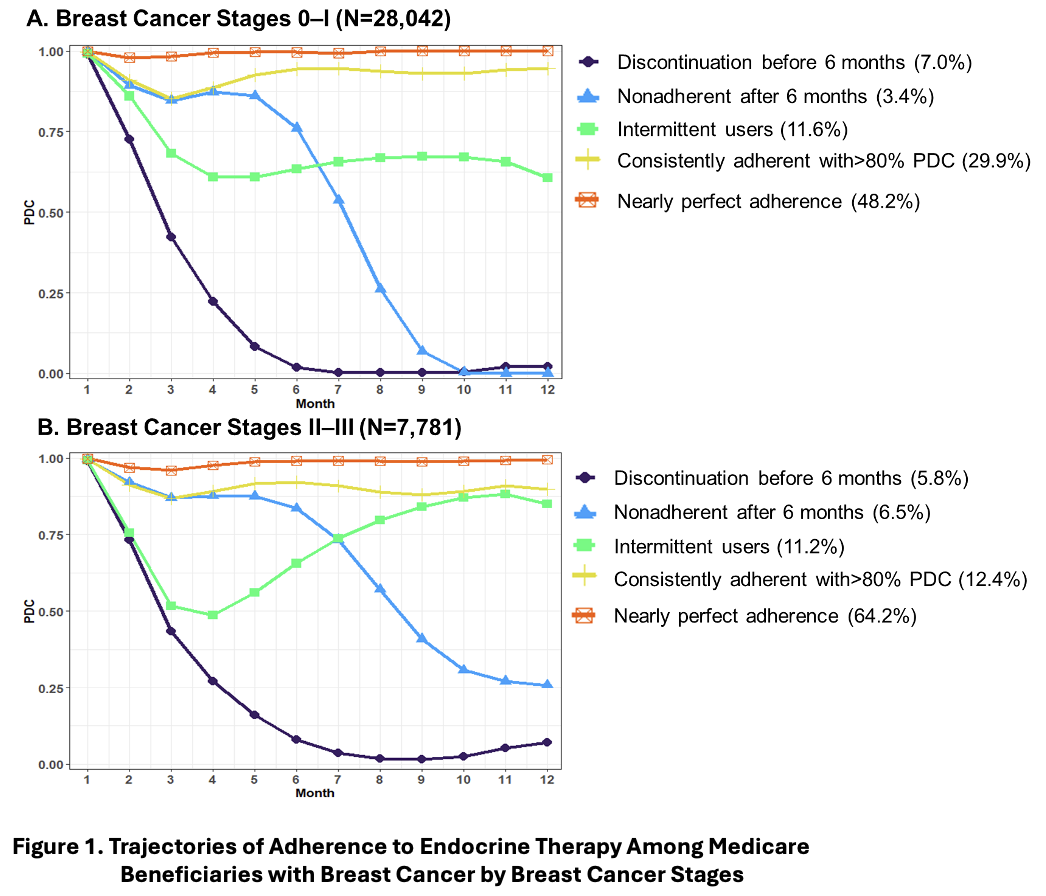
What can we do with this information? Since the women who discontinue endocrine therapy before 6 months in our study had a high risk of treated breast cancer recurrence, they may benefit from targeted interventions (e.g., medication therapy management follow-up) to identify potential side effects or barriers to maintenance of adherence. Since the women who discontinued endocrine therapy before 6 months made up a small proportion of the women in our study, interventions to help them adhere to their endocrine therapy would seem like a manageable undertaking.
References
1 SEER Program National Center for Health Statistics. Cancer Stat Facts: Breast cancer. (National Cancer Institute, 2019).
2 Burstein, H. J. et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol 32, 2255-2269 (2014). https://doi.org:10.1200/jco.2013.54.2258
3 Gradishar, W. J. et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 20, 691-722 (2022). https://doi.org:10.6004/jnccn.2022.0030
4 Partridge, A. H. et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26, 556-562 (2008). https://doi.org:10.1200/jco.2007.11.5451
5 Dezentjé, V. O. et al. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol 28, 2423-2429 (2010). https://doi.org:10.1200/jco.2009.25.0894
6 Weaver, K. E., Camacho, F., Hwang, W., Anderson, R. & Kimmick, G. Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low-income women. Am J Clin Oncol 36, 181-187 (2013). https://doi.org:10.1097/COC.0b013e3182436ec1
7 Makubate, B., Donnan, P. T., Dewar, J. A., Thompson, A. M. & McCowan, C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer 108, 1515-1524 (2013). https://doi.org:10.1038/bjc.2013.116
8 Font, R. et al. Influence of adherence to adjuvant endocrine therapy on disease-free and overall survival: a population-based study in Catalonia, Spain. Breast Cancer Res Treat 175, 733-740 (2019). https://doi.org:10.1007/s10549-019-05201-3
9 Barron, T. I., Cahir, C., Sharp, L. & Bennett, K. A nested case-control study of adjuvant hormonal therapy persistence and compliance, and early breast cancer recurrence in women with stage I-III breast cancer. Br J Cancer 109, 1513-1521 (2013). https://doi.org:10.1038/bjc.2013.518
10 Lee, Y. et al. Prescription Refill Gap of Endocrine Treatment from Electronic Medical Records as a Prognostic Factor in Breast Cancer Patients. J Breast Cancer 22, 86-95 (2019). https://doi.org:10.4048/jbc.2019.22.e14
11 McCowan, C., Wang, S., Thompson, A. M., Makubate, B. & Petrie, D. J. The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br J Cancer 109, 1172-1180 (2013). https://doi.org:10.1038/bjc.2013.464
12 Warren, J. L., Klabunde, C. N., Schrag, D., Bach, P. B. & Riley, G. F. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 40, Iv-3-18 (2002). https://doi.org:10.1097/01.Mlr.0000020942.47004.03
13 Potosky, A. L., Riley, G. F., Lubitz, J. D., Mentnech, R. M. & Kessler, L. G. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care 31, 732-748 (1993).
14 Mues, K. E. et al. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol 9, 267-277 (2017). https://doi.org:10.2147/clep.S105613
15 Nagin, D. S., Jones, B. L., Passos, V. L. & Tremblay, R. E. Group-based multi-trajectory modeling. Stat Methods Med Res 27, 2015-2023 (2018). https://doi.org:10.1177/0962280216673085
16 Nagin, D. S. & Odgers, C. L. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 6, 109-138 (2010). https://doi.org:10.1146/annurev.clinpsy.121208.131413
17 Schwarz, G. Estimating the dimension of a model. The annals of statistics, 461-464 (1978).
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in