ASXL1 mutation confers poor prognosis in PMF patients with low, but not high JAK2V617F allele burden
Published in Cancer
In addition to high-molecular risk (HMR) gene mutations, the low allele burden of the driver JAK2V617F has also been demonstrated to have a negative prognostic impact in primary myelofibrosis (PMF) patients(1-4). Nevertheless, further incorporation of the JAK2V617F allele burden and HMR gene mutations for prognostication of PMF patients has not been explored.
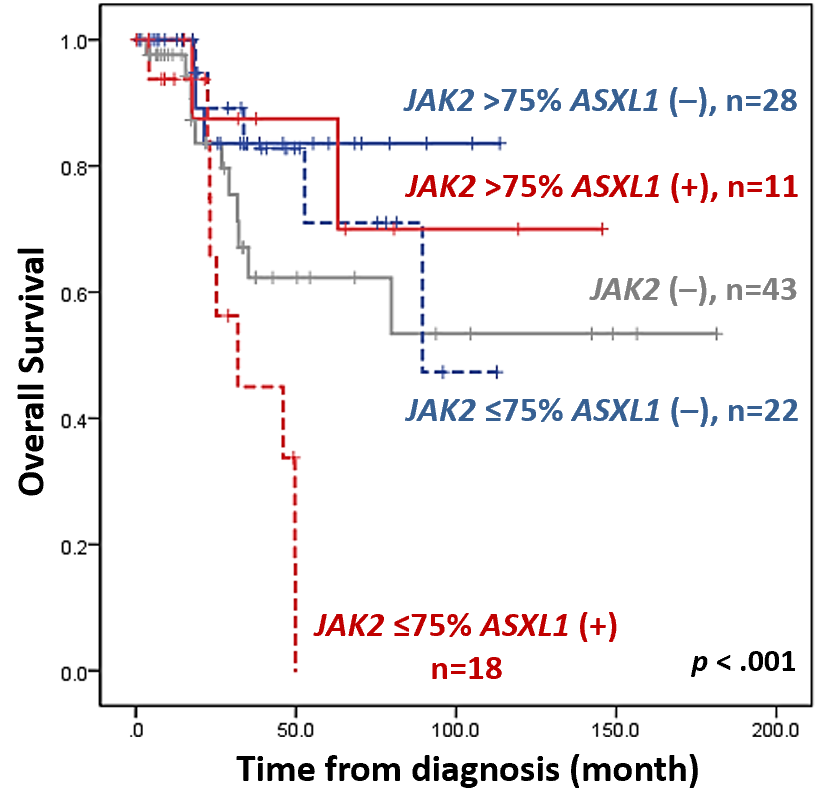
To address this, next-generation sequencing was adopted to profile mutation statuses of 54 myeloid neoplasm-relevant genes on bone marrow or peripheral blood cells in 122 PMF patients. JAK2V617F mutation was the most common driver mutation (65%), followed by CALR (18%) and MPL mutations (9%); and the most frequent associated mutation was ASXL1 (37%) mutation, followed by TET2 (16%), EZH2 (12%), DNMT3A (10%), and SRSF2 mutations (9%). Among the JAK2-mutated patients who had available information on the JAK2V617F allele burden, ASXL1 mutation conferred poor prognosis in patients with lower JAK2V617F allele burden but not in those with higher allele burden. Besides, patients with concurrent ASXL1 mutation and low JAK2V617F allele burden also had the shortest survival among the total cohort with JAK2-wild patients included (Figure). In multivariable analysis, concurrent ASXL1 mutation and low JAK2V617F allele burden was a significantly poor prognostic factor for leukemia-free and overall survival (OS), independent of the Dynamic International Prognostic Scoring System-Plus Score and statuses of other HMR mutations.
Tefferi et al first described the impact of the JAK2V617F allele burden on PMF patients’ survival: patients with JAK2V617F allele burden in the lower quartile had significantly reduced OS, compared with those with allele burden in the middle or upper quartile, or those without a mutant JAK2(3). This finding was supported by the study of Guglielmelli et al, within which a low JAK2V617F allele burden was confirmed as an independent adverse risk factor(4). Although the pathophysiologic mechanism underlying this correlation and the optimal cutoff value remain unknown, the contention that a lower JAK2V617F allele burden at diagnosis is associated with inferior survival in PMF patients is widely accepted. On the other hand, the role of a higher JAK2V617F allele burden in the MF phenotype is yet to be established.
The ASXL1 mutations are correlated with adverse prognosis in PMF. In a study of Guglielmelli et al, mutant ASXL1 and other three mutations (EZH2, SRSF2, and IDH1/2 mutations) were categorized as high-molecular risk mutations due to their detrimental effects on PMF patients’ survival(1). Recently, Tefferi et al identified seven adverse mutations by targeted deep sequencing and multivariable analysis(5). Among them, ASXL1 mutation was the most frequently detected and significantly associated with inferior survival.
As hematopoietic stem cell transplantation (HSCT) is currently the only curative treatment for PMF, the optimal timing and selection of candidates for transplant remain to be defined(6, 7). In this study, we identified a distinct patient population, characterized by having concurrent ASXL1 mutation and low JAK2V617F allele burden, who had a significantly shorter OS than others. Incorporation of the JAK2V617F allele burden to current risk stratification might provide complementary information to treatment decisions in the future(8).
https://doi.org/10.1038/s41408-020-00364-5
Reference
-
Guglielmelli P, et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: an international study of 797 patients. Leukemia. 2014;28(9):1804-10.
-
Tefferi A, et al. Driver mutations and prognosis in primary myelofibrosis: Mayo-Careggi MPN alliance study of 1,095 patients. Am J Hematol. 2018;93(3):348-55.
-
Tefferi A, et al. Low JAK2V617F allele burden in primary myelofibrosis, compared to either a higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia. 2008;22(4):756-61.
-
Guglielmelli P, et al. Identification of patients with poorer survival in primary myelofibrosis based on the burden of JAK2V617F mutated allele. Blood. 2009;114(8):1477-83.
-
Tefferi A, et al. Targeted deep sequencing in primary myelofibrosis. Blood Adv. 2016;1(2):105-11.
-
Kröger N. Stem cell transplantation in myelofibrosis. HemaSphere. 2018;2(S2):146-8.
-
Tiribelli M, et al. The role of allogeneic stem-cell transplant in myelofibrosis in the era of JAK inhibitors: a case-based review. Bone Marrow Transplantation. 2020;55(4):708-16.
-
Tefferi A, et al. Allogeneic hematopoietic stem cell transplant overcomes the adverse survival effect of very high risk and unfavorable karyotype in myelofibrosis. Am J Hematol. 2018;93(5):649-54.
Follow the Topic
-
Blood Cancer Journal

This journal seeks to publish articles of the highest quality related to hematologic malignancies and related disorders.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in