Behind the Paper: Potent antagonist for the MAS-related G protein-coupled receptor and its mouse ortholog with anti-inflammatory, anti- asthmatic, and anti-anaphylactic efficacy in human mast cells and mice
Published in Chemistry, Neuroscience, and Biomedical Research
Behind the Paper: Potent antagonist for the MAS-related G protein-coupled receptor and its mouse ortholog with anti-inflammatory, anti- asthmatic, and anti-anaphylactic efficacy in human mast cells and mice
Christa E. Müller* and Ghazl Al Hamwi
PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical & Medicinal Chemistry, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany
*Correspondence: christa.mueller@uni-bonn.de
Abstract
A unique class of antagonists with subnanomolar potency and high selectivity for the MAS-related G protein-coupled receptor X2 (MRGPRX2) and its putative mouse ortholog MRGPRB2 was developed by a focused, fragment-based approach. The best compound, PSB‑172656 (PSB, Pharmaceutical Sciences Bonn), was broadly evaluated showing drug-like properties and efficacy in human mast cells, where it inhibits MRGPRX2 agonist-induced degranulation. Moreover, it prevented inflammatory and anaphylactic reactions in mouse models.
The beginning: Search for the human adenine receptor
The project started more than 15 years ago, when we were looking for the human adenine receptor.1 Our group had been focused on purinergic signaling, developing tool compounds, e.g. for the G protein-coupled receptors (GPCRs) activated by the nucleosides adenosine (adenosine receptors) or by nucleotides (P2Y receptors). A study by Bender et al. (2002) had described the first GPCR that is activated by a nucleobase, namely adenine, expressed in rat. The receptor, initially designated adenine receptor-1 (rAdeR1) has later been identified as a member of the large family of MAS-related G protein-coupled receptors (MRGPRs), and is now known as MRGPRA. Later on, an ortholog was cloned from hamster (cAdeR1, MRGPRA), while two adenine receptors were cloned from mouse (mAdeR1 = MRGPRA10) and mAdeR2 = MRGPRA9).2 Several studies indicated that adenine-activated GPCRs existed also in humans. Therefore, we set out to identify the human adenine receptor(s). The GPCR family with the highest sequence similarity was the X-family MRGPRs, receptors that are primate-specific. Thus, we established cell lines and assay systems to study the interaction of adenine with the four MRGPRX subtypes, X1-X4. However, none of these GPCRs could be activated by adenine. In fact, the human adenine receptor has not been identified to this date.
Early screening campaign: Search for MRGPRX2 antagonists as tool compounds
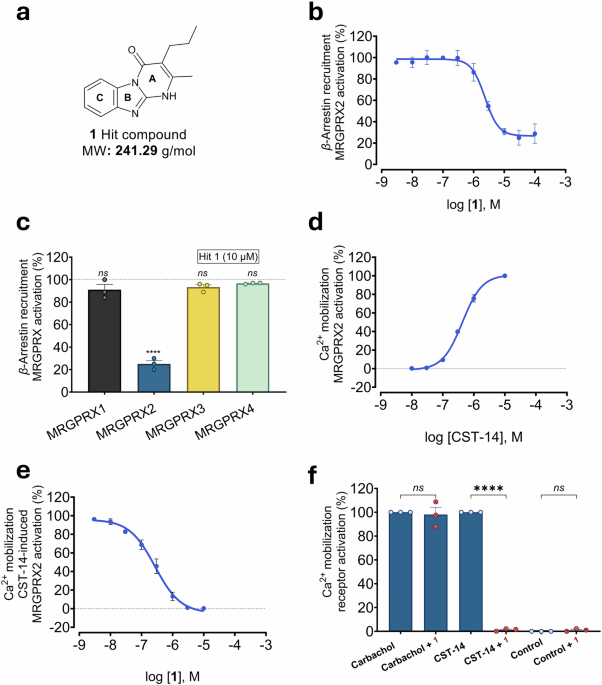
Since we had permanent cell lines and orthogonal assays in place, suitable for screening and characterizing MRGPRX2 ligands, and because no antagonists were available at the time, we set out to screen part of our in-house compound library for MRGPRX2 inhibition. In fact, we identified a small molecule hit which showed drug-like properties and appeared to be chemically tractable. After its characterization, we started a medicinal chemistry program to optimize its potency and other properties. This has been achieved by a multi-disciplinary team, including organic, medicinal and computational chemists and molecular pharmacologists, resulting in PSB-172656 with subnanomolar MRGPRX2-antagonistic potency and excellent properties as a tool compound (see Fig. 1).
Crucial discovery: MRGPRX2 induces degranulation of mast cells
When we learned that MRGPRX2 was expressed on mast cells. and might play an important role in mast cell degranulation,3 we extended our network of collaboration to experts in the field. In fact, PSB-172656 potently inhibited MRGPRX2 agonist-induced mast cell degranulation in a variety of mast cell models and also in native human mast cells, which are difficult to isolate, but could be obtained from breast skin tissue by our collaboration partners. In contrast, degranulation induced by IgE was not blocked proving the specificity of the effect. Importantly, the antagonist inhibited MRGPRX2 activation by structurally diverse agonists with very similar potency.
Mouse models: Inhibitory activity at the proposed mouse ortholog MRGPRB2
In 2015, Dong et al. published a seminal study, in which they proposed MRGPRB2 to act as a mouse ortholog of the human MRGPRX2.4 Since the mouse has the largest number of MRGPRs due to a gene expansion, it is possible that the functions of the human MRGPRX2 are shared by more than one receptor subtype. Nevertheless, the mouse MRGPRB2 showed significant overlap with the human MRGPRX2 with regard to ligands and functions. We subsequently established cell lines expressing the mouse MRGPRB2 and assays to test compounds. In fact, we observed that our antagonists, including PSB-172656, are similarly potent at the putative mouse ortholog, MRGPRB2, as at the human MRGPRX2. This is an essential activity of our compounds enabling subsequent studies in mouse models.
In fact, PSB-172656 showed efficacy in mouse models, preventing tracheal contractions, local allergic reactions, and systemic anaphylactic symptoms induced by MRGPRB2 activation.
Significance and outlook: Novel class of therapeutic drugs
PSB-172656 and its analogs are a unique class of highly potent MRGPRX2/B2 antagonists. In contrast to other MRGPRX2 antagonists, disclosed in the patent literature, which mostly represent peptidomimetic-like structures, our compounds are fragment-like small molecules, which may have pharmacokinetic and toxicological advantages. PSB-172656 is an excellent tool compound for mouse studies. It will serve as a lead structure for further optimization towards the development of drugs. There is a huge medical need for the therapy of so-far difficult-to-treat allergies of type VII (previously named pseudoallergic reactions). MRGPRX2 antagonists, as a monotherapy or in combination with other antiallergic drugs, will fill a gap e.g. for the treatment of certain types of anaphylaxis (including drug-induced allergic reactions), allergic rhinitis, urticaria, atopic dermatitis, rosacea, rheumatoid arthritis, chronic periodontitis, lung inflammation and fibrosis, ulcerative colitis, chronic prurigo, and a variety of other inflammatory and allergic diseases.2,5
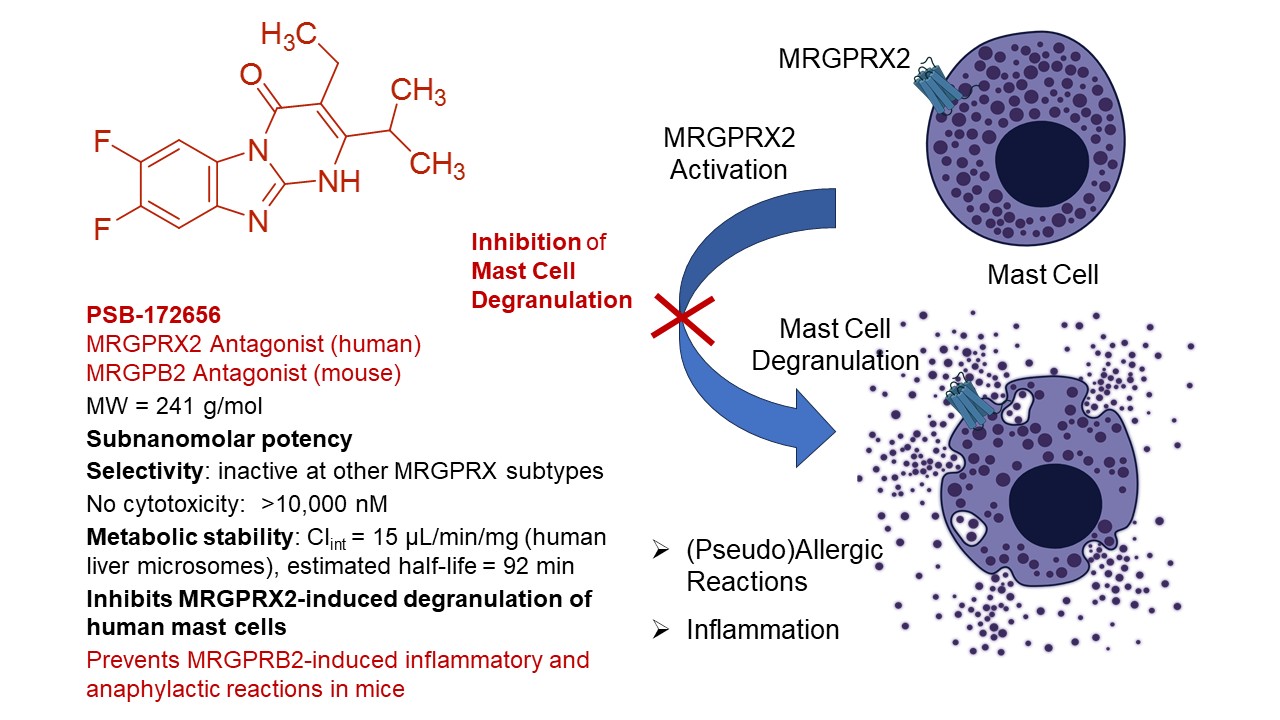
Fig. 1. Tool compound blocking human MRGPRX2 and its putative mouse ortholog MRGPRB2 showing in vitro and in vivo efficacy and suitable properties for further development of the scaffold to obtain a clinical candidate.
References
1. Thimm, D., Schiedel, A.C., Peti-Peterdi, J., Kishore, B.K., Müller. E. The nucleobase adenine as a signalling molecule in the kidney. Acta Physiol. (Oxf).213, 808-818 (2015)
2. Al Hamwi, G. et al. MAS-related G protein-coupled receptors X (MRGPRX): Orphan GPCRs with potential as targets for future drugs. Ther. 238, 108259 (2022).
3. Thapaliya, M., Avudhva, C.C.N., Amponnawarat, A., Roy, Saptarshi, Ali, H. Mast cell-specific MRGPRX2: a key modulator of neuro-immune interaction in allergic diseases. Curr. Allergy Asthma Rep. 21, 3 (2021).
4. McNeil, B.D. et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237-241 (2015).
5. Al Hamwi, G. et al. Proinflammatory chemokine CXCL14 activates MAS-related G protein-coupled receptor MRGPRX2 and its putative mouse ortholog MRGPRB2. Biol. 7, 52 (2024).
Follow the Topic
-
Signal Transduction and Targeted Therapy

This is an international, peer-reviewed, open-access journal publishing articles related to signal transduction in physiological and pathological processes, alongside signal transduction-targeted therapeutics in the form of biological agents and small molecular drugs used to treat human diseases.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in