Cambrian fossils will get on your nerves
Published in Ecology & Evolution

Half a billion-year-old nerves
About a decade ago, the thought that fossils could preserve the brains of 500-million-year-old animals would cause disbelief and raise more than a few eyebrows. The fossil record is essentially composed of highly resistant, usually biomineralized parts of extinct organisms, such as the shells of clams and snails or the bones of dinosaurs. By contrast, nervous tissues are only made of cells and a good dose of fat, so the odds for them to survive millions of years in sediments would deter even the most audacious gamblers. Experiments aiming at quantifying and characterizing decay further consolidated this idea: some organic tissues are simply too delicate for the conventional fossil record. Amongst internal organs, only the gut – a chemical reactor filled with bacteria, food, and sometimes sediment – may be replicated by minerals during body decomposition, and thus leave a trace in fossils.
Flash-forward to today, over a dozen instances of neurological preservation in fossils have been reported from lower to mid-Cambrian marine strata of Canada, China, Greenland, and the USA (Figure 1). These fossilized nervous tissues are preserved as organic carbon films typical of so-called Burgess Shale-type deposits, and they show a strong taxonomic bias, as most examples concern euarthropods. Fossil nerves in other animal groups are as-yet much rarer with only a single occurrence to date for an annelid worm, a priapulid worm, and a comb jelly. Palaeoneurological data has significantly helped clarify the head segmentation and overall body plan evolution in euarthropods, complementing the detailed information provided by their external exoskeletal morphology. However, euarthropods were just as extraordinarily diverse in the Cambrian as they are today, and reconstructing their relationships relative to modern representatives is a challenge that requires as much data as the fossil record can muster.
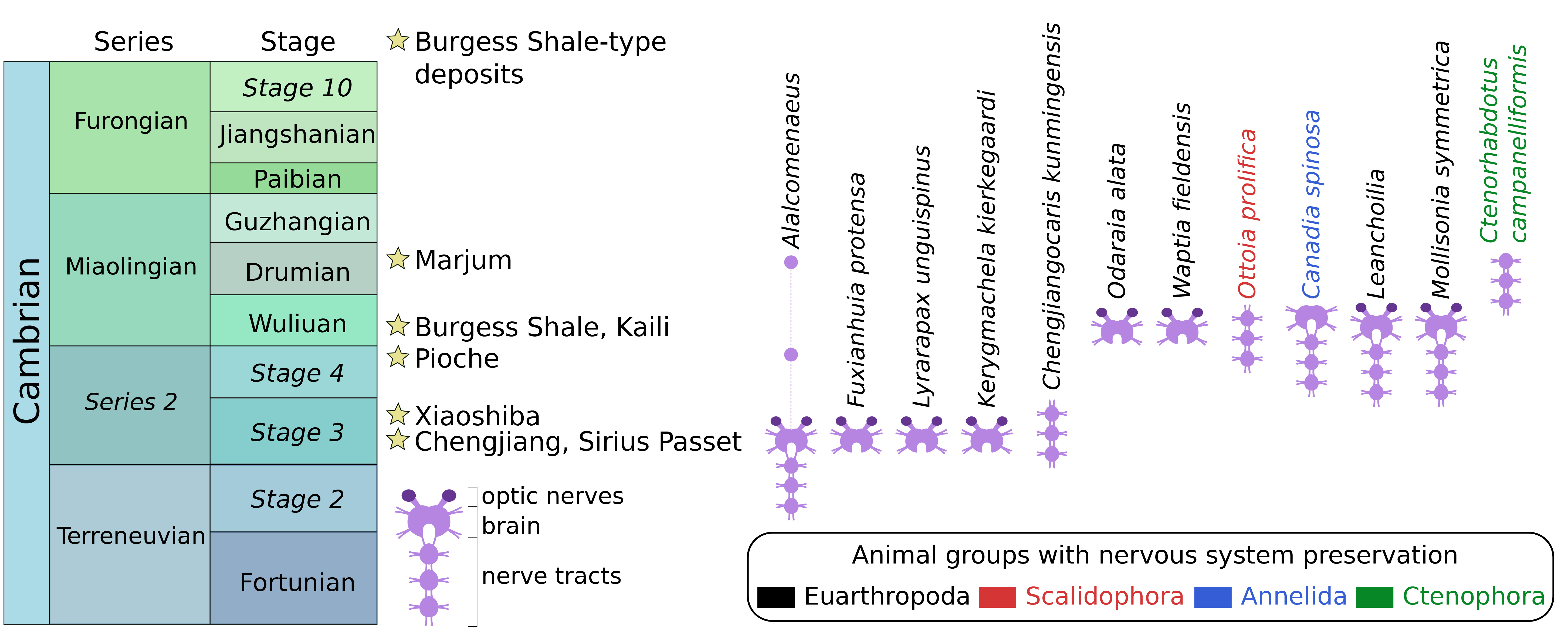
Figure 1. Stratigraphic distribution of Cambrian Burgess Shale-type deposits that preserve at least one example of a fossilized nervous system. Updated from Ortega-Hernández et al. (2019).
Mollisonia symmetrica has guts… and brains!
There are numerous groups of bizarre Cambrian euarthropods, several of which occur globally and thus have come to occupy an important place in discussions about the origin of the phylum. All-time favorites, such as the radiodont Anomalocaris and the megacheiran Leanchoilia, come to mind. Other groups appear much less flamboyant in the eyes of non-specialists by virtue of their excessive rarity, insufficient aesthetic appeal, or lack of imagination-fuelling anatomical attributes such as raptorial limbs or impressive mouthparts. Until recently, mollisoniids were amongst the nobodies of the Cambrian Explosion, despite being known from Burgess Shale-type deposits around the world. With a taxonomic diversity of only seven species, most of which are only known from a handful of specimens, and a simple dorsal morphology consisting of a head shield, multisegmented body and posterior shield, mollisoniids have historically received little attention from the public and specialists alike. However, the recent description of chelicerate-like appendages in Mollisonia, including possible chelicerae-like mouthparts, has finally put the spotlight on these extinct animals, which are now regarded as some of the oldest relatives of spiders, scorpions, and mites.

Figure 2. Specimen of Mollisonia symmetrica (Smithsonian Institution – USNM 305093), photographed under cross-polarized illumination dry (showing exoskeleton), low angle illumination (showing relief), and cross-polarized illumination wet (showing internal anatomy).
In our study, we tackled mollisoniid internal anatomy and evolutionary significance thanks to the discovery of the fossilized nervous system in Mollisonia symmetrica from the mid-Cambrian (ca. 508-million-year-old) Burgess Shale in British Columbia (Figure 2). In this species, the dorsal exoskeleton overlies a gut tract with segmentally disposed pairs of large glands, a widespread and ecologically significant feature in Cambrian euarthropods. A few select specimens preserve neurological structures below the digestive system and expressed as carbonaceous films. The nervous system consists of delicate optic nerves connected to a pair of large and bulbous lateral eyes, an indistinctly preserved brain mass underneath the head shield, and a ventral nerve cord with serially repeated ganglia that match the segmentation of the dorsal exoskeleton in the trunk (Figure 3). The fossils demonstrate that the posterior shield has three nerve bundles associated with it, supporting the hypothesis of its three-segmented nature. The inconspicuousness of the brain in Mollisonia is puzzling, considering that it innervated no less than seven pairs of highly differentiated cephalic limbs. One could expect that such a crowding of cephalic appendages would have resulted in the evolution of a well-developed synganglion, a highly condensed brain mass similar to those of extant and extinct horseshoe crabs, but no such feature is visible. Despite the observation of fine anatomical details in our material, an incomplete preservation of the brain remains possible, especially considering the limited number and particularly small size of the studied specimens. An alternative is that Mollisonia illustrates a decoupling between the evolution of appendages and that of the nervous system in early chelicerates.
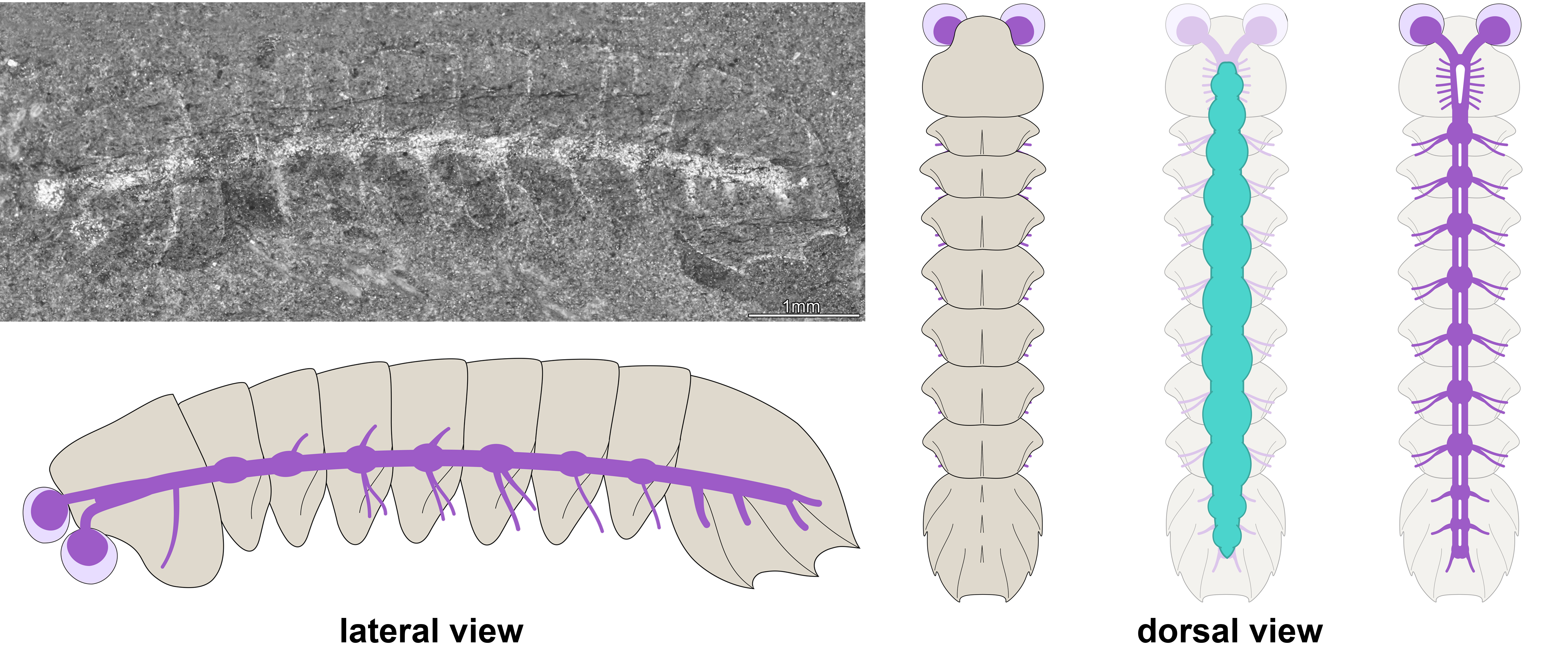 Figure 3. Fossilized central nervous system and morphological reconstruction of Mollisonia symmetrica. Note that the nervous system (purple in the diagram) is expressed as a continuous reflective film connected with the eyes, and which runs throughout the entire body. The digestive system (green in the diagram) is located above the nervous system, as observed in modern euarthropods.
Figure 3. Fossilized central nervous system and morphological reconstruction of Mollisonia symmetrica. Note that the nervous system (purple in the diagram) is expressed as a continuous reflective film connected with the eyes, and which runs throughout the entire body. The digestive system (green in the diagram) is located above the nervous system, as observed in modern euarthropods.
Mozaic evolution and the origin of chelicerate body plan
The discovery of the fossilized central nervous system in Mollisonia symmetrica is exciting not only because it provides yet another demonstration of the extraordinary preservation potential in Burgess Shale-type deposits, but also because of the phylogenetic position of this taxon at the base of the chelicerate lineage. Chelicerates are among the most diverse invertebrate animals on our planet today (approximately 120,000 described species), and they play a critical role as predators in terrestrial ecosystems, including our homes. Reconstructing their origin and early evolutionary history during the Cambrian is of the utmost significance for euarthropod palaeobiology.
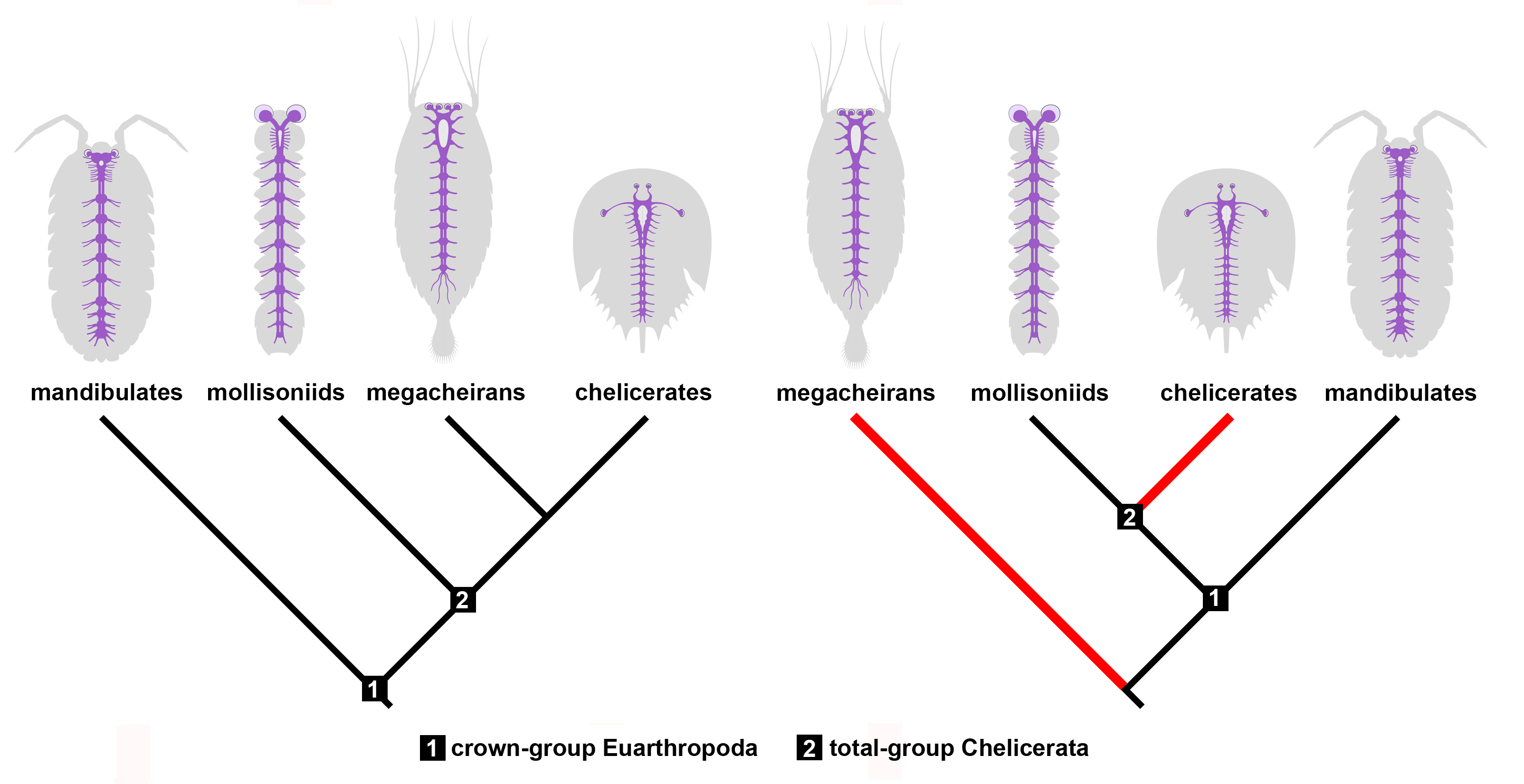
Figure 4. Implications of Mollisonia’s nervous system for reconstructing chelicerate evolution. Mollisonia represents an early chelicerate based on the structure of its appendages. If megacheirans, or great appendage euarthropods, are close relatives of chelicerates as suggested by their similar neuroanatomy (left panel), then mollisoniids reveal the ancestral organization of the nervous system. However, if megacheirans are not closely related to chelicerates (right panel), the similarities observed in the nervous system observed between these two groups are the result of convergent evolution (red lines).
Our study on Mollisonia symmetrica suggests that the ancestral nervous system of chelicerates had a relatively simple organization compared to extant representatives. For example, extant horseshoe crabs and arachnids feature eye doublets, a cephalic synganglion with an elongate oesophageal opening, and in some species a nerve cord only represented by elongate connectives in the posterior body. Mollisonia lacks all these neurological traits, which suggests that it embodies many ancestral characteristics (Figure 4). On the other hand, its complex appendicular anatomy suggests a close relationship with modern chelicerates. These conflicting phylogenetic signals are exacerbated by comparisons with the Megacheira, another group of probable Cambrian relatives of chelicerates. Megacheirans, such as Leanchoilia and Alalcomenaeus, share several neurological features with extant chelicerates, even though they share few aspects of their dorsal exoskeleton and appendage morphology, with the main similarity being the presence of a set of raptorial first appendages. In short, megacheirans are closer to modern chelicerates than mollisoniids from a neuroanatomical perspective, but the opposite is true from an appendicular perspective. This conundrum creates two alternatives. One in which mollisoniids and megacheirans occupy progressively closer positions to chelicerates as informed by the evolution of their nervous systems, and another in which only mollisoniids are related to chelicerates, but megacheirans have evolved similar neurological characters independently through convergence (Figure 4). Both options carry different implications for the affinity of megacheirans – as either chelicerate relatives or occupying a more distant position the euarthropod tree of life – but also indicate that there is a conflict between the information observed in either internal or external anatomy. Given the rarity of complete nervous systems in Cambrian fossils, this represents one of the first cases in which different parts of the animal tell different evolutionary histories, with no definitive answer at the time as to which one is more likely to be true at the moment. This apparent paradox also suggests that mosaic evolution was at play in the early history of chelicerates. Despite these complications, the Cambrian fossil record continues to regularly produce highly informative specimens that significantly contribute to a more profound understanding of how early animals lived and evolved. With the discovery of new fossil localities on the rise, it is only a matter of time until the next fossil comes along and answers our most pressing questions, if only to open a few more in the process.
Javier Ortega-Hernández and Rudy Lerosey-Aubril
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in