Catalytic Enantioselective Reductive Alkynylation of Amides Enables One-Pot Syntheses of Pyrrolidine, Piperidine and Indolizidine Alkaloids
Published in Chemistry

The important of efficiency in the total synthesis of natural products
Today, developing highly efficient total synthesis of natural products is not only the major goal in organic synthesis, but also crucial for the renaissance of natural products as drug candidates[1]. Pyrrolidine, piperidine, and indolizidine alkaloids are prevalent among the plant and animal kingdoms. Among ant venom alkaloids and over 800 alkaloids isolated from skin
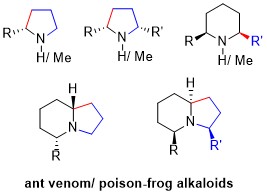
piperidine, and indolizidine alkaloids, an
anticancer antipode, and a medicinal agent.
of poison-frogs as of 2005, most possess a stereogenic unbranched a-alkyl or a cis or trans-a,a’-dialkyl pyrrolidine/piperidine motif (Figure 1). Such structural motifs also found in many medicinal agents. The scarcity from natural sources and significant biological activities possess by these natural products render them significant targets for the total synthesis.
The state-of-the-art and challenge for developing highly efficient asymmetric total syntheses of pyrrolidine, piperidine, and indolizidine alkaloids
The tremendous endeavors over last five decades have resulted in numerous innovative synthetic approaches towards pyrrolidine, piperidine, and indolizidine alkaloids. However, highly efficient asymmetric total syntheses remain rare and lack of versatility. For example, the synthesis of (+)-monomorine I (A-8), an indolizidine alkaloid first isolated from Monomorium pharaonis L. as a pharaoh ant trail pheromone in 1973, and then from the extracts of the ant Myrmicaria melanogaster from Brunei, has attracted considerable attention. A Web of Science search using simultaneously “monomorine” and “synthesis” as key words reveals 105 papers and dissertations including three in 2023[2,3]. Among them, the shortest and most efficient asymmetric total synthesis (5 steps, 15.9% overall yield, and 6 steps, 26.2% overall yield)[2,4], representing the state-of-the-art of this field, and reflexing the challenge for developing highly efficient asymmetric total syntheses.
Key step as a key to achieve synthetic efficiency
Key reactions such as cycloadditions/annulations, one-pot sequential/tandem/cascade reactions, and multicomponent reactions have long been recognized to play a key role for achieving high synthetic efficiency in the total synthesis. Very recently, Cernak and coworkers reported a computer-aided key step identification, which resulted in a three-step total synthesis of (–)-stemoamide[5,6]. However, the synthesis relies on known key reactions, highlighting the demand for novel key reactions/steps for highly efficient total synthesis of natural products, new medicines, and agrochemicals.
Catalytic Enantioselective Reductive Alkynylation of Teriary Aliphatic Amides: A key reaction for the efficient asymmetric synthesis of pyrrolidine, piperidine, and indolizidine alkaloids
Our longstanding interest in the total synthesis of alkaloids (Angew. Chem. Int. Ed. 2016, 55, 4064-4068; Nature Commun. 2020, 11: 5314) and in the direct transformation of amides (Angew. Chem., Int. Ed. 2018, 57, 11354–11358; Angew. Chem., Int. Ed. 2021, 60, 8827–8831; Angew. Chem., Int. Ed. 2021, 60, 26604–26609; Sci. Adv. 2022, 8, eade3431), enabled us to identify the chemoselective, catalytic asymmetric reductive alkynylation of unbranched aliphatic tertiary amides as the key step for the efficient asymmetric synthesis of pyrrolidine, piperidine, and indolizidine alkaloids (Fig. 2). However, this remains an unresolved challenge.
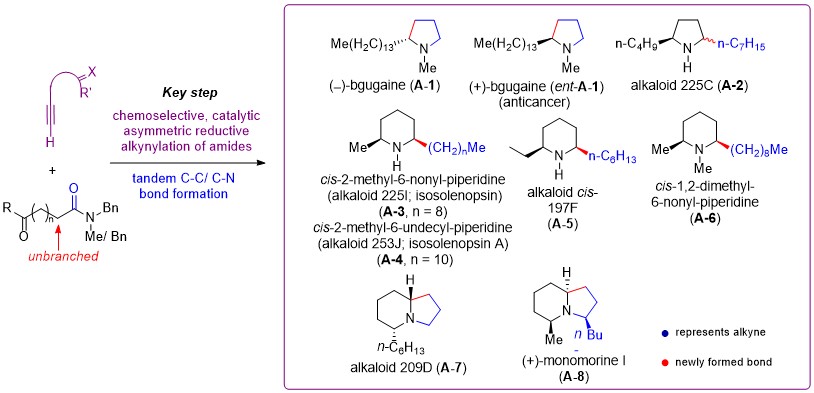
We started our investigation by developing an Ir/Cu/N-PINAP catalyzed highly enantioselective and chemoselective reductive alkynylation of a-unbranched aliphatic amides (Scheme 1).
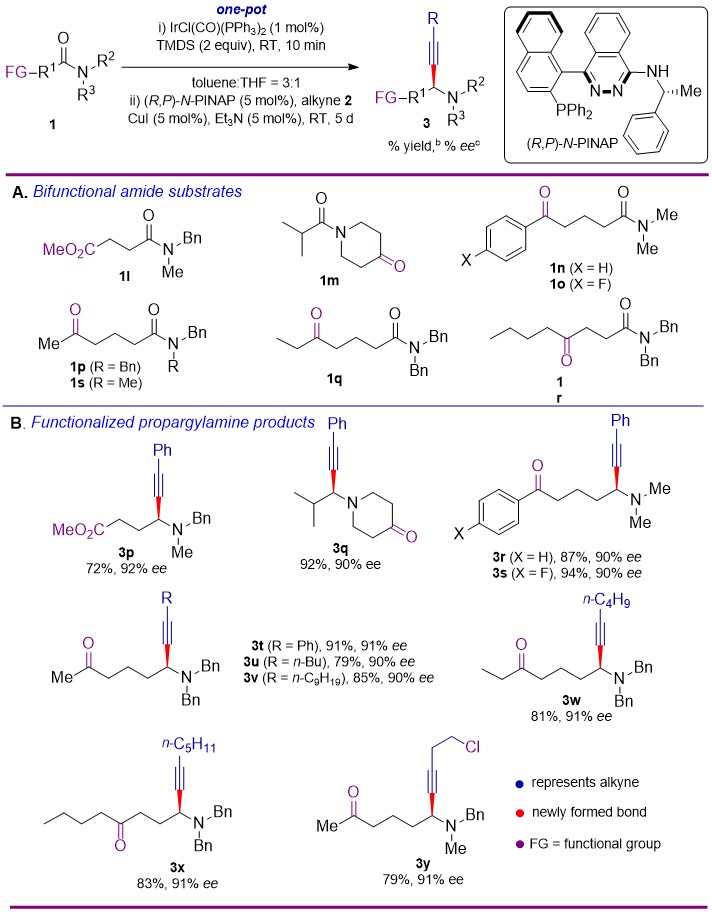
chemoselectivity and functional group tolerance
By merging this key reaction with Pd-catalyzed tandem reactions that include hydrogenation of triple bond of the alkynyl moiety, N-debenzylation, cleavage of ketone protecting group, and reductive alkylation of amines, we have developed a one-pot asymmetric total synthesis of ant venom/poison-frog alkaloids from amides and alkynes. Using this powerful methodology, we have achieved the catalytic enantioselective total syntheses of 8 alkaloids and an anticancer antipode with 90-98% ee. As outlined in Scheme 2, because the amide and alkyne are available both in one-step from commercially available compounds, our catalytic asymmetric total synthesis of monomorine I (A-8) represents the shortest (three steps in total) and most efficient asymmetric total synthesis to date of this bicyclic alkaloid bearing three stereogenic centers.
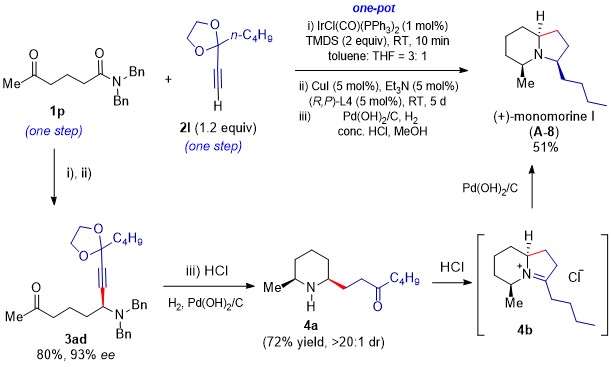
Scheme 2. One-pot, catalytic enantioselective total synthesis of indolizidine alkaloid monomorine I (A-8).
References
[1] Huang, P.-Q., Yao, Z.-J. & Hsung, R. P. (eds). Efficiency in Natural Product Total Synthesis (John Wiley & Sons, Inc., 2018).
[2] Nakagawa, H., Fuwa, H. Au-catalyzed stereodivergent synthesis of 2,5-disubstituted pyrrolidines: total synthesis of (+)-monomorine I and (+)-indolizidine 195B. Chem. Commun., 59, 10121-10124 (2023).
[3] Gu, Z.-Y., Li, W.-D., Li, Y.-L., Cui, K., Xia, J.-B. Selective Reductive Coupling of Vinyl Azaarenes and Alkynes via Photoredox Cobalt Dual Catalysis. Angew. Chem. Int. Ed. 62, e202213281 (2023).
[4] McManus, J. B., Onuska, N. P. R., Nicewicz, D. A. Generation and Alkylation of α‑Carbamyl Radicals via Organic Photoredox Catalysis. J. Am. Chem. Soc. 140, 9056-9060 (2018).
[5] Lin, Y.-F., Zhang, R., Wang, D. & Cernak, T. Computer-aided Key Step Generation in Alkaloid Total Synthesis. Science 379, 453-457 (2023).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in