Cation controlled rotation in anionic pillar[5]arenes and its application for fluorescence switch
Published in Chemistry
The manual manipulation of motions at the molecule level is one of hot topics in the field of chemistry. Molecular rotors have various potential applications in many aspects including molecular devices, medicine, asymmetric catalysis, and smart materials, which are derived from their controllable rotations.1-3 Therefore it is of great significance to develop novel rotor systems with controllable manners. Pillar[n]arenes, an emerging class of macrocycles, have a unique planar chirality, in which two stable conformations pR and pS could interconvert by oxygen-through-the-annulus rotations of their hydroquinone rings (Fig. 1a).4-5 Hence pillar[n]arenes could be considered as promising rotor platforms.
From 1H NMR results, it was noticed that the signal of methylene group in the rims of sodium carboxylate pillar[5]arene (WP5-Na, Fig. 1a) was split into two doublets, while in ammonium carboxylate pillar[5]arene (WP5-NH4, Fig. 1a) case it was a singlet (marked in green, Fig. 1b), indicating slower rotations of hydroquinone moieties in WP5-Na and faster rotations in WP5-NH4.6 We supposed such distinct kinetic properties may attribute to impacts of counterions Na+ and NH4+ on rotational barriers of hydroquinone rings in WP5. This discovery provided us possibilities to construct switchable rotations in WP5 by means of cation switch. In addition, we inferred controlling rotary speed of hydroquinone rings in WP5 would thus result in fluorescence switch, due to restricted rotations reduced energy dissipations, and thus enhanced emission intensity. In this work, we investigate the effect of various counterions on rotations of hydroquinone rings in WP5, establishing a controllable rotary system, and its applications in fluorescence switch and anti-counterfeiting inks are further developed (Fig. 1c). 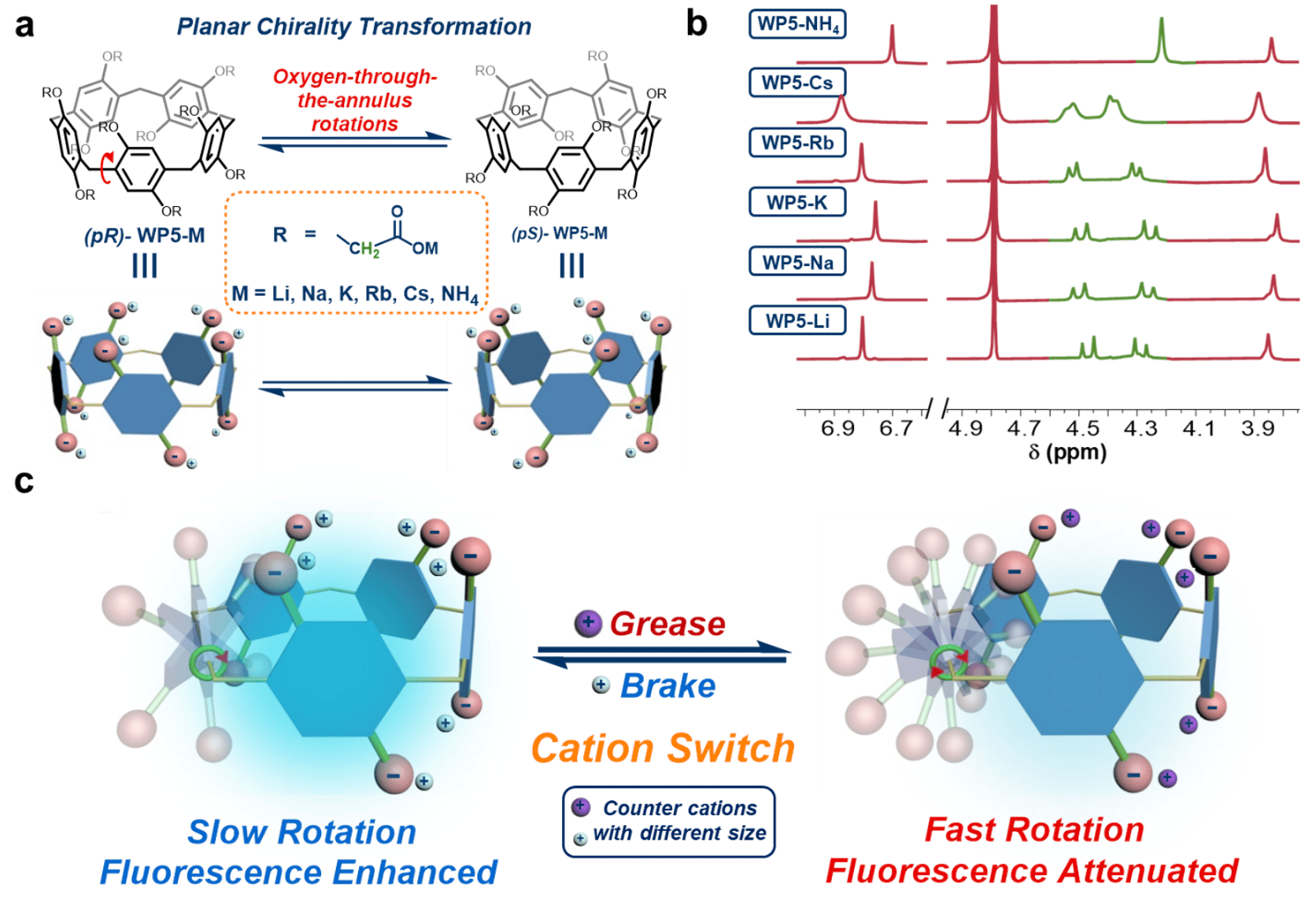
Fig. 1 (a) Illustration of planar chirality transformation of WP5-M. (b) 1H NMR spectra of WP5-M in D2O (10.0 mM, 298 K), peaks of methylene groups are shown in green. (c) Cartoon Illustrations of cation-modulated switchable rotary motion of WP5-M. For simplification, only one rotary phenolic ring of WP5 is shown.
To explore the impact of counterions on rotations of hydroquinone units in anionic pillar[5]arenes, carboxylate pillar[5]arenes with various monovalent counterions were chosen (WP5-M, Fig. 1a). WP5-M were then subjected to variable temperature (VT) NMR studies, and a rotational barrier (DG¹) of 18.23, 17.55, 15.95, 15.68, and 15.06 kcal/mol was revealed based on Eyring plots.6Interestingly, a linear line was obtained when we tried to correlate the experimental rotational barriers with the radius r of corresponding cations (R2 = 0.99, Fig. 2a). It decreases in rotational barrier DG¹ as the ionic radius increases (negative slope). Although it was a failure to measure the rotational barrier of WP5-NH4 owing to the limitation of the freezing point of deuteroxide, a qualitative result could be drawn that WP5-NH4 possessed the lowest rotational barrier.
Besides, a linear relationship between experimental ΔS¹ and ΔH¹ was observed, which was a typical enthalpy-entropy compensation (R2 = 1.00, Fig. 2b).7 It suggested the existence of enhanced binding force (enthalpy favored) in transition stats (TS) from WP5-Li to WP5-Cs, which would lead to reduction of configurational freedom (entropy decrease). Therefore, we speculated that this downward trend of rotational barriers could be interpreted as following: in rotary process, there was a strong electronic repulsion between carboxylate anions in the rims and the electron-rich cavity of pillar[5]arenes (Fig. 2c). Cation acting as grease, could insert between anions and cavities, involving coulomb interactions with anions and cation-p interactions with cavities, by which the disfavored repulsion could be impaired. Ascribing to a size-specific mechanism that was ubiquitous in macrocyclic host-guest systems, larger counter cations might have stronger interactions, leading to an enhanced lubricant effect (Fig. 2c). Furthermore, although radii of NH4+and K+ were similar (1.43 Å and 1.38 Å, respectively), WP5-NH4 had a lowest rotational barrier, which could be attributed to additional hydrogen bond between ammonium ion and carboxylate oxygen.
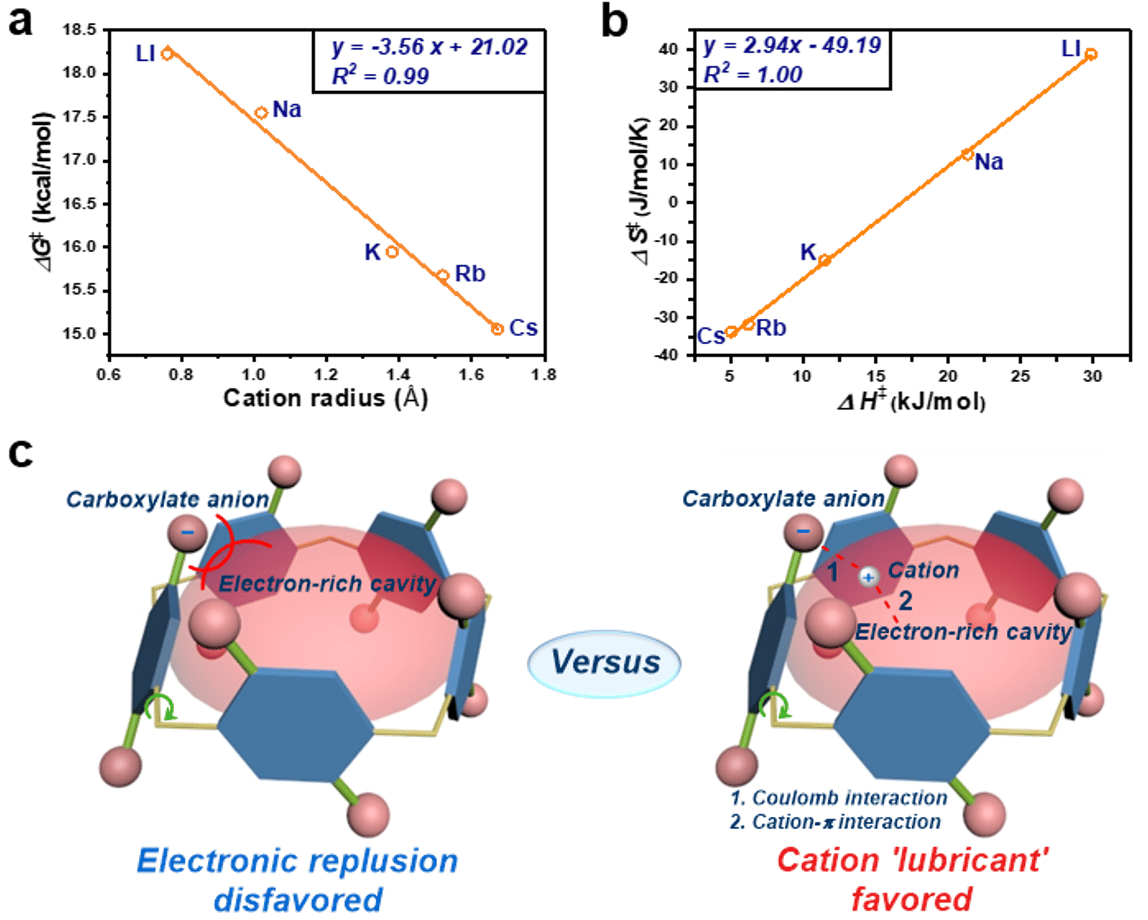
Fig. 2 (a) Correlation of the rotational barrier ΔG¹exp with corresponding cation radius. (b) Enthalpy-entropy compensation plot of WP5-M. (c) Illustration of electron repulsion between carboxylate anion and electron-rich cavity versus cation lubricant effect.
Theoretical calculations were next carried out for validating our above-mentioned hypothesis. Stoddart's TS model for pillar[n]arenes was employed,8 and the stage TS1 and TS2 were evaluated by the potential energy surface scanning (PESS). The highest energy barriers among two rotational processes (TS1 and TS2b) were evidently reduced from WP5-Li, WP5-Na, WP5-K to WP5-NH4, which was in accordance with experimental results. A decrease in the non-bonded distance between cations and vertical phenolic rings in m- position (WP5-Li: 2.87 Å; WP5-Na: 2.74 Å; WP5-K: 2.35 Å; WP5-NH4: 2.37 Å) was observed in sampled structures from PESS, which indicated the enhanced cation-p interactions (Fig. 3). Meanwhile, hydrogen bonding between ammonium ion and carboxylate oxygen of WP5-NH4 was significant, which could be contributed to the lowest rotational barrier of WP5-NH4 among these anionic pillar[5]arenes (Fig. 3).
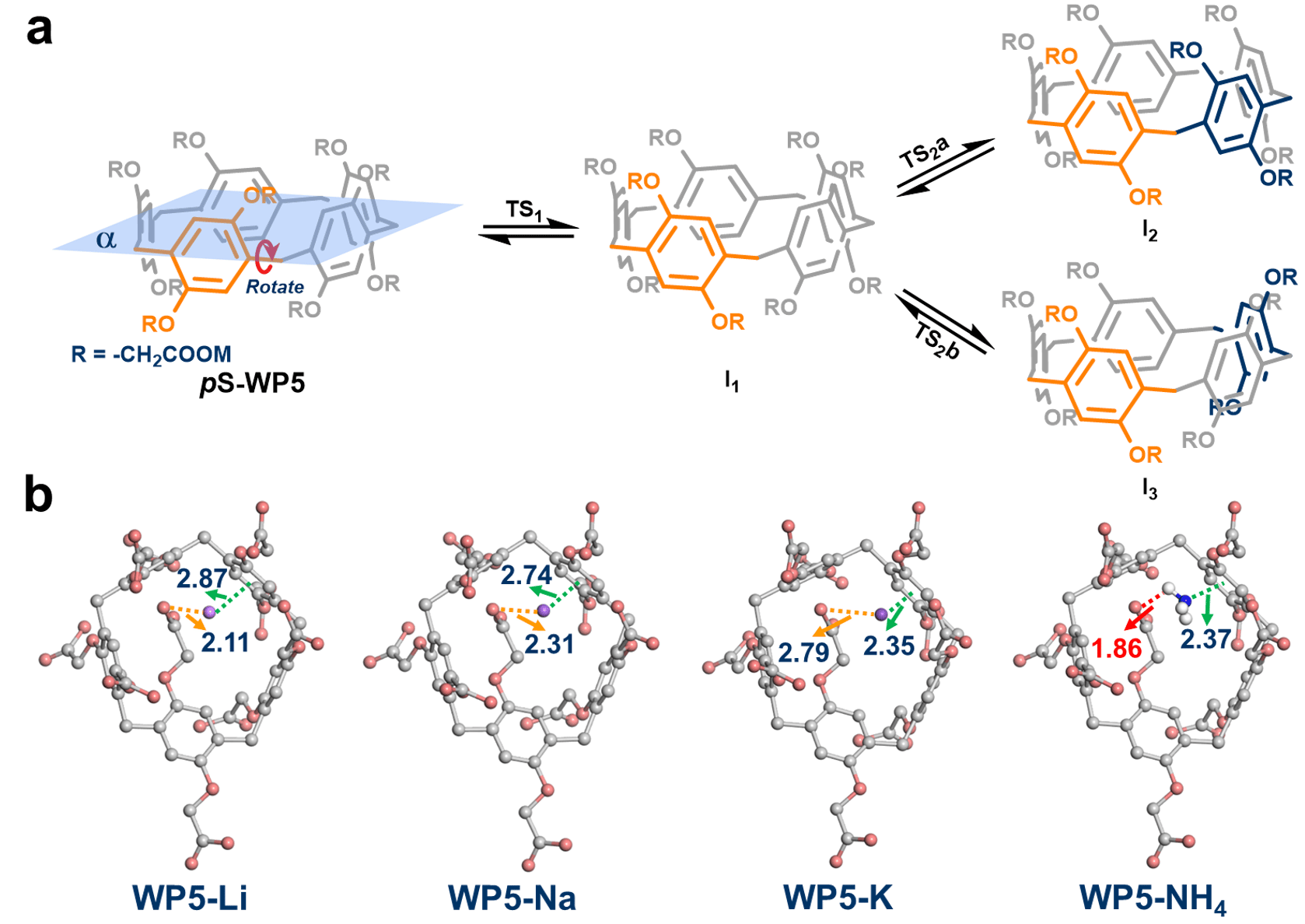 Fig. 3 (a) Partial pathways of transformation between its pS and pR conformers of WP5-M. (b) Configurations extracted from potential energy surface scanning when hydroquinone ring rotated to the plane a. The non-bonded distances are in units of Å.
Fig. 3 (a) Partial pathways of transformation between its pS and pR conformers of WP5-M. (b) Configurations extracted from potential energy surface scanning when hydroquinone ring rotated to the plane a. The non-bonded distances are in units of Å.
Finally, switchable rotary motions of rotors were explored. The acceleration/deacceleration of rotors was accomplished by means of cation exchange (Fig. 4a-4c), and these findings further allowed the fluorescence switch (Fig. 4d), which was applied as anti-counterfeiting inks (Fig. 4e).
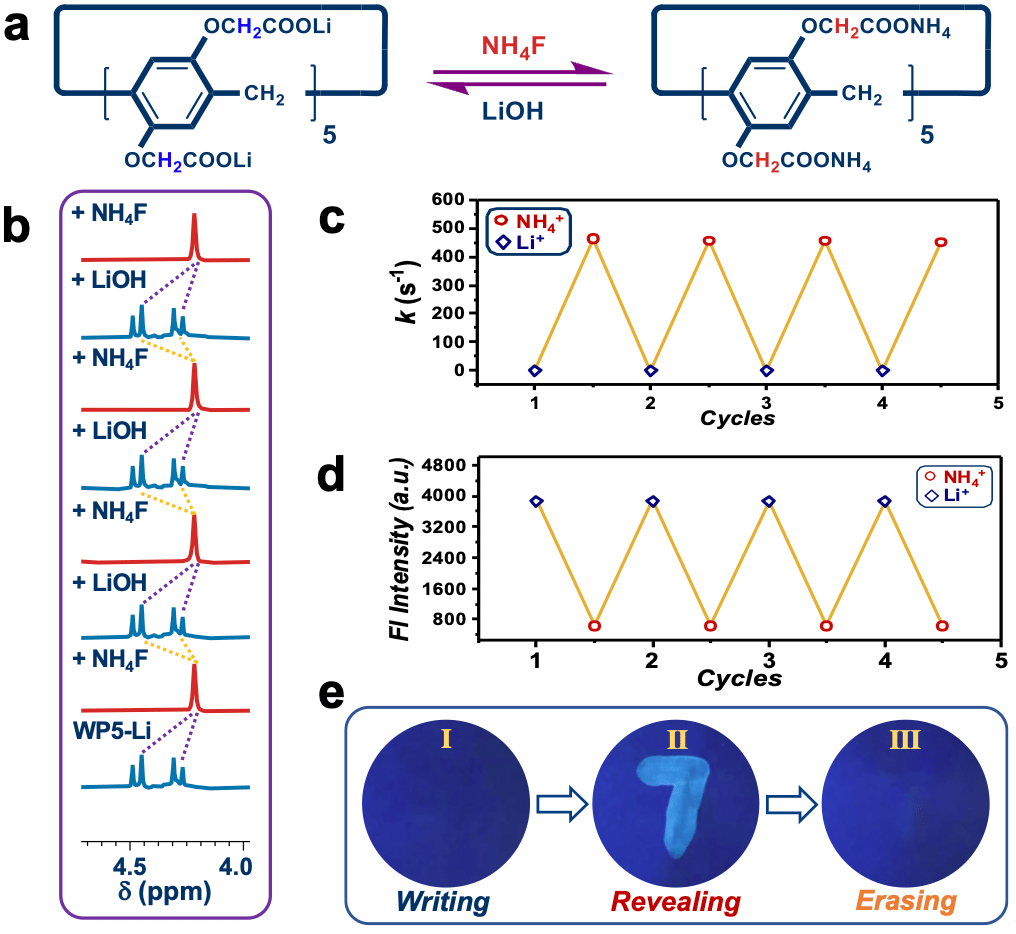 Fig. 4 (a) Cation switch between Li+ and NH4+. (b) Partial NMR of switching control of rotors. (c) Switching of rate for WP5 in D2O at 298 K with multiple cycles of cation exchange. (d) Fluorescence response of WP5 (5.0 mM) at 471 nm upon multiple cycles of cation switch between Li+ and NH4+ in D2O (lex = 365 nm). (f) Revealing and erasing information with WP5 inks.
Fig. 4 (a) Cation switch between Li+ and NH4+. (b) Partial NMR of switching control of rotors. (c) Switching of rate for WP5 in D2O at 298 K with multiple cycles of cation exchange. (d) Fluorescence response of WP5 (5.0 mM) at 471 nm upon multiple cycles of cation switch between Li+ and NH4+ in D2O (lex = 365 nm). (f) Revealing and erasing information with WP5 inks.
References
- Kassem, S., van Leeuwen, T., Lubbe, A. S., Wilson, M. R., Feringa, B. L., Leigh, D. A. Artificial molecular motors. Chem. Soc. Rev.46, 2592-2621 (2017).
- van Leeuwen, T., Lubbe, A. S., Štacko, P., Wezenberg, S. J., Feringa, B. L. Dynamic control of function by light-driven molecular motors. Nat. Rev. Chem. 1, 0096 (2017).
- Mei, J., Leung, N. L., Kwok, R. T., Lam, J. W., Tang, B. Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 115, 11718-11940 (2015).
- Ogoshi, T., Yamagishi, T. A., Nakamoto, Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 116, 7937-8002 (2016).
- Fa, S., Kakuta, T., Yamagishi, T., Ogoshi, T. Conformation and Planar Chirality of Pillar[n]arenes. Chem. Lett. 48, 1278-1287 (2019).
- Abraham, R. J., Fisher, J., Loftus, P. Application of NMR Spectroscopy. Introduction to NMR Spectroscopy. (John Wiley & Sons Ltd:New York, 1988).
- Liu, L., Guo, Q.-X. Isokinetic Relationship, Isoequilibrium Relationship, and Enthalpy-Entropy Compensation. Chem. Rev. 101, 673-695 (2001).
- Strutt, N. L., Schneebeli, S. T., Stoddart, J. F. Stereochemical inversion in difunctionalised pillar[5]arenes. Supramol. Chem. 25, 596-608 (2013).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in