CD24: A Cell Surface Marker for Anastasis in Melanoma
Published in Cancer and Cell & Molecular Biology

Context
Since 2014, I have been pursuing the idea that certain polymorphic human germline variants affect proximal signaling in both cancer and immune cells, thereby playing a crucial role in determining individual-specific cancer progression and contributing to the individual-to-individual variability (aka individuality) of therapy outcomes. In 2020, as the research working group leader of the "Immune Signaling Pathways in Autoimmunity and Cancers" group at the Dermatology Department of the University Medical Center, Göttingen, I was working on developing a series of in vitro functional assays aimed at reducing reliance on transgenic mice while addressing a key question: how do cancer-associated germline variants impact the tumor microenvironment and influence immunotherapy outcomes?
A given cancer-associated variant of uncertain significance can not only enhance oncogenic signaling within tumor cells but also influence the immune system in a manner that supports tumor progression. For example, a variant may limit the infiltration of tumor-reactive T cells or dampen the anti-tumor CD8 T cell response by directly suppressing TCR signaling in response to tumor antigens. In this regard, our previous work on the germline variant rs351855-G/A, also known as the FGFR4 p.Gly388Arg variant, which creates a membrane-proximal STAT3 docking site (Ulaganathan et al., Nature, 2015), was the first to establish the concept that a cancer-associated germline variant can directly influence CD8 T cell infiltration in the tumor microenvironment. This was demonstrated using SNV knock-in genetically engineered mouse models of breast cancer and non-small cell lung cancer (Kogan et al., JCI, 2018). We soon realized there were many cancer-associated germline variants with similar molecular alterations on membrane-proximal signaling (Ulaganathan VK, Scientific Reports. 2020) that are currently considered variants of uncertain significance (VUS) in the field of applied genomics. Studying these variants by generating SNV knock-in mice for each one is time-consuming and expensive.
To tackle this issue, we genetically engineered B16-F10 melanoma cells using sleeping beauty plasmid constructs to constitutively express and present Ovalbumin (OVA) on their surface. Using these cells and genetically engineered OT-I MHC Class I restricted CD8 T cells, we developed a method to functionally characterize potential phosphotyrosine-altering SNVs (which we refer to as pTyr-SNVs) in a rapid and less expensive in vitro setting (Ulaganathan VK & Vasileva MH, J Genet Genomics. 2023), minimizing the need for genetically modified animals and instead utilizing genetically engineered cell lines. Given that B16-F10 tumors are considered poorly immunogenic, we screened for surface molecules that might inhibit T cell receptor (TCR) signaling in OT-I T cells.
Behind the Paper
While analyzing various molecules through flow cytometry-based surface expression assays, we inadvertently included CD24 in our antibody panel. At first we thought CD24 surface expression is an artefact, as the distinct subpopulation that showed positivity for CD24 was located in the quadrant of side scatter (SSC) high and forward scatter (FSC) low area – typically considered dead cells in flow cytometry dot plots. However, the isotype IgG control did not show a similar binding pattern. Additional investigation by immunoblot and QPCR confirmed expression of CD24 in B16-F10 cells, leading us to ask why surface expression is different from the total cytoplasmic expression. So we decided to investigate the biological significance of this finding. This insight sparked the development of a Master’s thesis project, very successfully conducted by Ms. Martina H. Vasileva, which eventually got published in the journal Apoptosis (Vasileva MH et al, Apoptosis. 2024). In this paper, Vasileva MH reports the finding that CD24 marks melanoma cells destined to exhibit anastasis. Here we explored the biochemical and molecular properties of the CD24+ subpopulation in B16-F10 cells, and later corroborated the results in the C57BL/6-derived YUMM5.2 melanoma cell line as well.
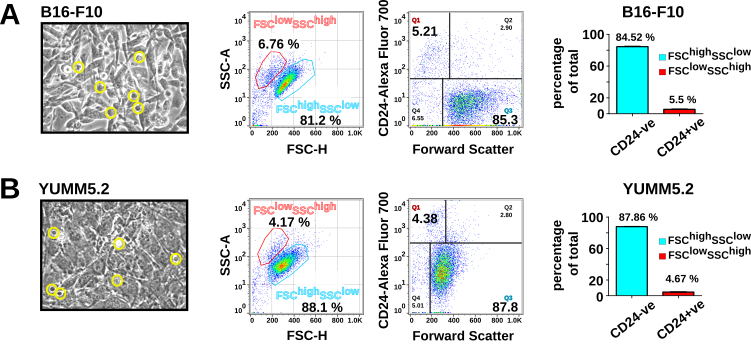
In this paper, we reveal that FSC-veSSC+veCD24+ve melanoma cells exhibited all hallmarks of apoptosis yet retained the ability to regenerate colonies in 3D agarose colony formation assays, a key indicator of tumorigenic potential. The concept of "anastasis" — the process by which cells that have initiated apoptosis are able to recover and survive — has emerged as a fascinating area of cancer research. Our recent work advances this field by identifying CD24 as a novel surface marker for anastasis in melanoma cells, a breakthrough that could have important implications for understanding melanoma metastasis. More than 90% of non-adherent floating FSClowSSChighCD24+ve melanoma cells exhibited classical features of apoptosis, including both surface and nuclear markers. Yet, surprisingly, these seemingly apoptotic cells demonstrated metabolic activity and proliferative capacity, including the ability to grow independently in soft agarose. Interestingly, these subpopulations also express PD-L1 on their surface, indicating a potential role in immunomodulation (see Fig. 3). Our findings suggest that these apoptotic FSClowSSChighCD24+ve subpopulations have the ability to reverse apoptosis, highlighting CD24 as the first reported surface marker for anastasis in melanoma cells.
This discovery opens up exciting avenues for further research into the role of anastasis in cancer progression and metastasis, and it holds promise for novel therapeutic approaches that target this survival mechanism in melanoma. This unexpected finding also raises many intriguing scientific questions, which we have discussed in our paper.
References:
Vasileva MH, Bennemann A, Zachmann K, Schön MP, Frank J*, Ulaganathan VK*. CD24 flags anastasis in melanoma cells. Apoptosis. 2024 Aug 13. doi: 10.1007/s10495-024-01990-1. Online ahead of print. PMID: 39136818
Ulaganathan VK*, Vasileva MH. A strategy for uncovering germline variants altering anti-tumor CD8 T cell response. J Genet Genomics. 2023 May;50(5):353-361. doi:10.1016/j.jgg.2023.01.001. Epub 2023 Jan 20. PMID: 36690075.
Ulaganathan VK*. TraPS-VarI: Identifying genetic variants altering phosphotyrosine based signalling motifs. Sci Rep. 2020 May 21;10(1):8453. doi: 10.1038/s41598-020-65146-2. PMID: 32439998
Kogan D, Grabner A, Yanucil C, Faul C, Ulaganathan VK*. STAT3-enhancing germline mutations contribute to tumor-extrinsic immune evasion. J Clin Invest. 2018 May 1;128(5):1867-1872. doi: 10.1172/JCI96708. Epub 2018 Apr 3. PMID: 29438108.
Ulaganathan VK*, Ullrich A*. Membrane-proximal binding of STAT3 revealed by cancer-associated receptor variants. Mol Cell Oncol. 2016 May;3(3):e1145176. doi:10.1080/23723556.2016.1145176.
Ulaganathan VK*, Sperl B, Rapp UR, Ullrich A*. Germline variant FGFR4 p.G388R exposes a membrane-proximal STAT3 binding site. Nature. 2015 Dec 24;528(7583):570-4. doi:10.1038/nature16449. Epub 2015 Dec 16. PMID: 26675719.
* Corresponding author(s)
Follow the Topic
-
Apoptosis

A peer-reviewed journal devoted to basic and clinically-oriented investigations into programmed cell death.
Your space to connect: The Cancer in understudied populations Hub
A new Communities’ space to connect, collaborate, and explore research on Cancers, Race and Ethnicity Studies and Mortality and Longevity!
Continue reading announcement





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in