Dietary methionine restriction: is there a dark side in cancer treatment?
New discoveries are always made by chance
The earliest exploration of the relationship between methionine and cancer dates back to 1959. The observation that cancer cells have an increased requirement for methionine compared to normal cells led to a hypothesis that restricting methionine intake could potentially inhibit cancer growth. Since then, numerous studies have established methionine restriction (MR) as an anti-cancer dietary regimen that suppresses tumor proliferation/progression and improves responses to various anti-cancer therapies 1-6.
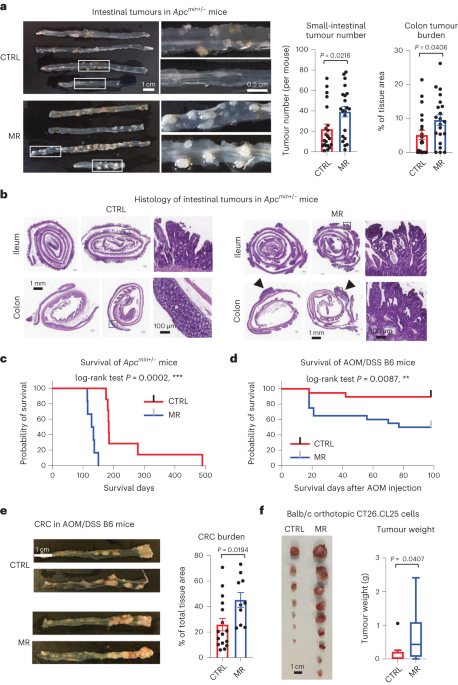
Following this line of research, our lab recently showed that different human liver cancer cell lines have drastically different responses to MR depending on the status of HNF4α, a transcription factor that regulates numerous hepatic genes including sulfur amino acid enzymes 7. To test the importance of HNF4α in dictating tumor sensitivity to MR in vivo, we followed up with an MR experiment in an HNF4α positive genetic cancer model, Apcmin+/- mice, which have high spontaneous formation of multiple intestinal neoplasia (min) and short health-span. Based on previous findings from our and other labs, we expected that MR will reduce the formation of intestinal adenoma and extend the health-span. To our surprise, methionine-restricted Apcmin+/- mice displayed aggravated tumor progression and dramatically shortened symptom-free survival compared with those fed with a control high-methionine diet.
Confirming the phenomenon in mice and humans
To confirm the seemly counterintuitive findings in Apcmin+/- mice, we repeated MR feeding in two additional cancer models, an Azoxymethane (AOM)/Dextran sodium sulfate (DSS)-induced colorectal cancer model in C57BL/6J mice and a syngeneic orthotopic CT26 colon cancer model in Balb/c mice. In both models, we observed increased tumor growth/progression by MR pre-feeding. These observations told us that MR promotes tumor growth/progression independently of cancer models.
We further excluded possible diet-specific impacts, as Apcmin+/- mice fed with a chow diet containing 0.4% L-methionine had significantly increased intestinal tumor number/burden and shortened health-span compared to those fed with a custom-made methionine-enriched chow diet containing 1.3% L-methionine.
More importantly, we found an inverse relationship between low protein intake (a proxy for methionine intake) and cancer risk in humans.
Complex and interesting mechanisms
We observed the intriguing tumor-promoting effect of MR in Apcmin+/- mice first in late 2018. While we were scrambling in confirming this unexpected observation, a couple of studies on the importance of methionine in T cell activation and differentiation were published in 2019 and early 2020 8,9. These reports reminded us that mice used in our study, including Apcmin+/- mice, C57BL/6J mice, and Balb/c mice, are all immunocompetent in contrast with immunodeficient mice utilized in previous xenograft and PDX studies. So, MR could potentially impair host anti-immunity, thereby promoting tumor growth. Indeed, we found that low methionine diets inhibit tumor growth in immunodeficient mice yet promoting tumor formation in immunocompetent mice. Moreover, MR diet feeding reduced T cell abundance and dampened anti-tumor immunotherapy efficacy in immunocompetent mice. These effects were observed in multiple tumor models and suggest that MR-induced immune suppression promotes tumor growth and limits the response to immunotherapy.
Then, how does MR suppress host anti-tumor immunity? Two studies in 2020 both reported the involvement of methionine in epigenetic regulation of immune cell activation/differentiation through production of SAM, a major methyl-donor for DNA/histone methylation 9,10. But epigenetics is for sure not the only mechanism, especially we found that dietary supplementation of L-cysteine, a sulfur amino acid acting downstream of SAM, rescues MR-induced resistance to anti-tumor immunotherapy.
Role of Gut Microbiota:
Given the importance of gut microbiota in regulating the host immune system and response to anti-tumor therapies, we speculated that MR may affect anti-tumor immunity through modulation of the gut microbiota.
We first showed that MR profoundly influences the gut microbiota in both tumor-bearing and tumor-free mice. Particularly, MR depleted Akkermansia muciniphila while increasing Bifidobacterium, both are major types of gut commensal bacteria frequently associated with colon carcinogenesis and therapeutic outcomes. We then demonstrated that gut microbiota indeed mediates the impact of dietary MR on T cell activation and tumor progression. For instance, dietary MR did not affect T cell abundance and tumor growth in germ-free immunocompetent mice. Moreover, transplantation of fecal microbiota from methionine-restricted tumor-free animals was sufficient to suppress T cell activation and enhance tumor growth in tumor-bearing recipient mice.
Role of Gut Microbial H2S Production:
In search of how gut microbiota mediates the impact of MR on anti-tumor immunity, we made another counterintuitive but intriguing observation, as our data showed that defective fecal H2S production is partially responsible for the impaired immune cell activation observed in methionine-restricted mice. Fecal metabolite analyses revealed that reduced fecal H2S producing activity is a major metabolic defect in MR diet fed mice. Administration of GYY4137, a slow-release H2S donor, significantly reduced the tumor burden and/or sensitized tumors to anti-PD1 immunotherapy in methionine-restricted tumor-bearing immunocompetent mice but not in immunodeficient mice. Finally, in a proof-of-concept experiment, reduced H2S production from gut microbiota directly impaired anti-tumor immunity and dampened the response of subcutaneously grafted tumors to anti-tumor immunotherapy. Together, our findings highlight the significance of H2S as a mediator of immune cell activation and its potential as a therapeutic target in cancer treatment.
Take-home massage
Our study uncovers a non-cell autonomous mechanism by which dietary methionine affects tumor growth and anti-tumor immunity through gut microbiota-mediated activation of immune cells. Specifically, we showed that MR, a dietary regimen protecting against metabolic diseases and aging, negatively impacts anti-tumor immunity and the therapeutic response to anti-tumor immunotherapy. Therefore translationally, MR may be beneficial for immunocompromised cancer patients but be detrimental to immunocompetent individuals. Conversely, methionine supplementation may enhance anti-tumor immunity in immunocompetent patients but promote tumor growth in immunocompromised patients. Our study also proposes the use of H2S chemical donors to enhance anti-tumor immunity and immunotherapy efficacy. Additionally, our study suggests that the net effect of dietary methionine on cancer growth depends on the relative dependence of cancer cells vs immune cells on this nutrient in the tumor microenvironment, which may vary at different stages of tumor development. Collectively, our seemingly counterintuitive discoveries reflect the intricate trade-offs between tumor promoting and immune activation impacts of methionine and H2S. Understanding the complex relationship between dietary factors, gut microbiota, and immune system is crucial for developing strategies to improve anti-cancer therapy in the future.
References
1 Guo, H. et al. Therapeutic tumor-specific cell cycle block induced by methionine starvation in vivo. Cancer Res 53, 5676-5679 (1993).
2 Poirson-Bichat, F., Gonfalone, G., Bras-Goncalves, R. A., Dutrillaux, B. & Poupon, M. F. Growth of methionine-dependent human prostate cancer (PC-3) is inhibited by ethionine combined with methionine starvation. Br J Cancer 75, 1605-1612, doi:10.1038/bjc.1997.274 (1997).
3 Sinha, R. et al. Dietary methionine restriction inhibits prostatic intraepithelial neoplasia in TRAMP mice. Prostate 74, 1663-1673, doi:10.1002/pros.22884 (2014).
4 Ables, G. P., Hens, J. R. & Nichenametla, S. N. Methionine restriction beyond life-span extension. Ann N Y Acad Sci 1363, 68-79, doi:10.1111/nyas.13014 (2016).
5 Wang, Z. et al. Methionine is a metabolic dependency of tumor-initiating cells. Nat Med 25, 825-837, doi:10.1038/s41591-019-0423-5 (2019).
6 Gao, X. et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 572, 397-401, doi:10.1038/s41586-019-1437-3 (2019).
7 Xu, Q. et al. HNF4alpha regulates sulfur amino acid metabolism and confers sensitivity to methionine restriction in liver cancer. Nat Commun 11, 3978, doi:10.1038/s41467-020-17818-w (2020).
8 Sinclair, L. V. et al. Antigen receptor control of methionine metabolism in T cells. Elife 8, doi:10.7554/eLife.44210 (2019).
9 Roy, D. G. et al. Methionine Metabolism Shapes T Helper Cell Responses through Regulation of Epigenetic Reprogramming. Cell Metab 31, 250-266 e259, doi:10.1016/j.cmet.2020.01.006 (2020).
10 Bian, Y. et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature 585, 277-282, doi:10.1038/s41586-020-2682-1 (2020).
Follow the Topic
-
Nature Metabolism

This journal publishes work from across all fields of metabolism research that significantly advances our understanding of metabolic and homeostatic processes in a cellular or broader physiological context, from fundamental cell biology to basic biomedical and translational research.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in