Enabling transport and metabolism in synthetic cells
Published in Bioengineering & Biotechnology, Chemistry, and Microbiology
Building a synthetic cell from molecular components represents one of the biggest scientific and technological challenges of the 21st century, with biotechnological and biomedical implications, including specialized drug delivery1,2, chassis for biosensing and metabolic engineering3, enzyme replacement technologies4, nanoreactors for therapeutics5, carbon fixation6 and others. But foremost, it will provide blueprints for constructing cells and give mechanistic insight in complex life-like processes. Synthetic cells can be constructed by assembling engineered cellular “ingredients” (biomolecules or entire modules), designed to mimic vital cellular functions in a membrane-bounded compartment. Controlled spatiotemporal integration of ingredients like genetic material, machinery for protein expression, synthesis and/or transport of building blocks, homeostasis strategies and energy conversion will lead to life-like systems that will grow, replicate its genome and divide (Figure 1).
Our work focuses on the development of energy modules to drive and sustain essential cellular processes. Previously, we developed systems that produce NAD(P)H or adenosine triphosphate (ATP) for powering redox7 or drive metabolic reactions or membrane transport8. The ATP system can fuel volume regulation8 and lipid biosynthesis9, and recycle adenine nucleotides for enabling reactions in other compartments, akin what mitochondria do in living cells. Now, we present synthetic vesicles with a simple pathway to store energy in the form of a proton electrochemical gradient (proton motive force, PMF) across the membrane. This form of metabolic energy is needed for the sustained transport of amino acids, sugars and other building blocks, and can be used for pH and ion homeostasis.
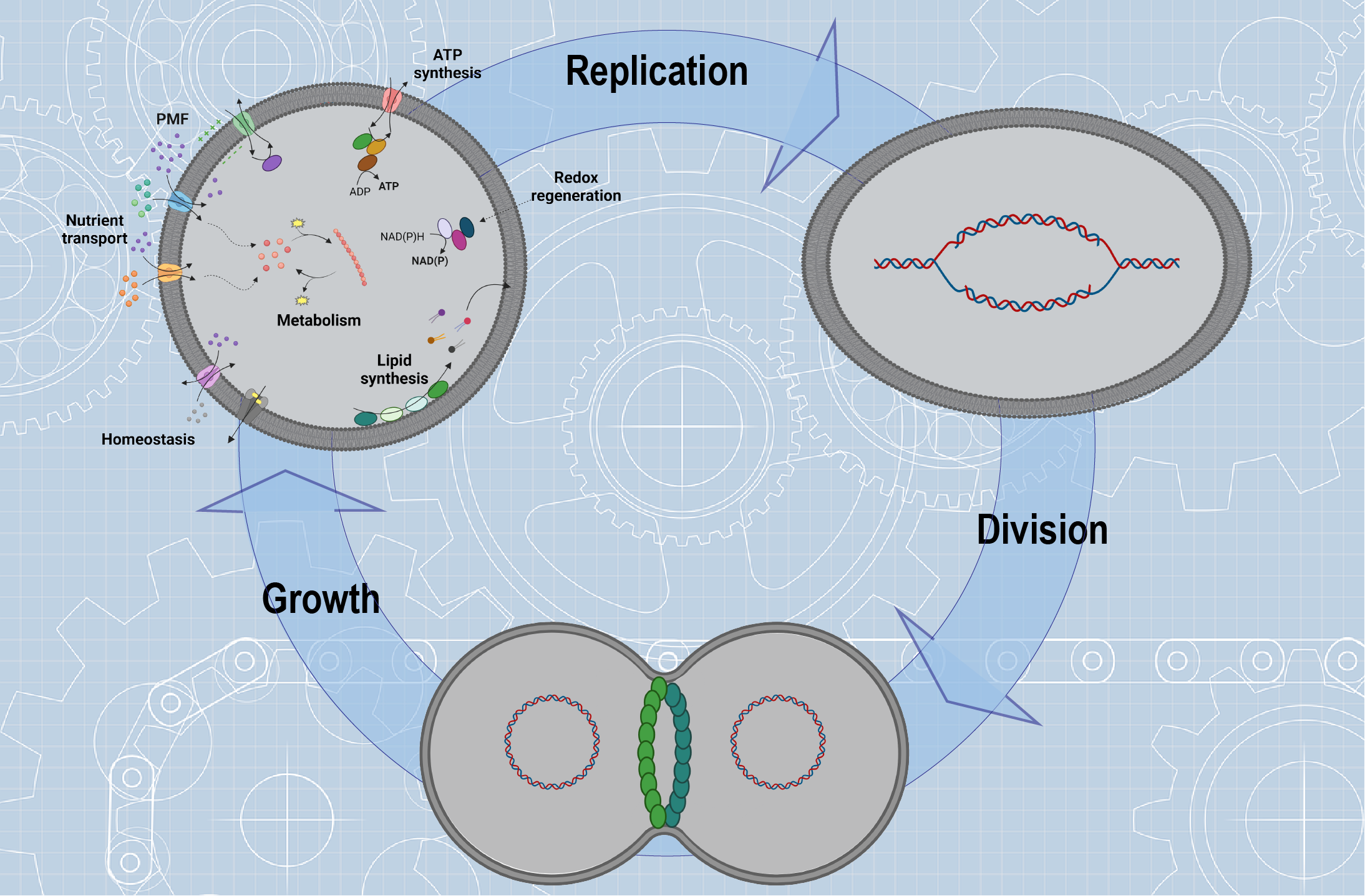
Figure 1. Cartoon showing the construction of a synthetic cell using the bottom-up synthesis approach. Building a synthetic cell requires compartments, machineries for protein expression, building blocks synthesis and/or transport, homeostasis, metabolism, growth, DNA replication and division. The energy for the organization and function of these ingredients into a synthetic cell comes from ATP and proton motive force (PMF). Created with BioRender.com.
Energy, transport and metabolism: brick by brick construction
What makes cells distinctively more complex than man-made devices is that their content cannot be described as a set of static molecules and reactions, but the molecules form a highly dynamic and intertwined network that enables sustainable and autonomous functioning. In the paper “Chemiosmotic nutrient transport in synthetic cells powered by electrogenic antiport coupled to decarboxylation”, we functionally connect a series of reactions that are essential in living cells, but the molecular components come from different cell types and have been integrated into a new blueprint.
We reconstituted a pathway for the decarboxylation of the dicarboxylic acid L-malate, which includes a transport step that generates an electrical potential across the membrane and an enzymatic reaction in which a proton is consumed inside the vesicles, leading to the formation of a pH gradient (Figure 2a, b). Like in a living cell, we coupled the formation of the membrane potential and pH gradient to the uptake of two key metabolites, i.e. the amino acid glutamate and the carbohydrate lactose. In fact, the reaction network enabled a more than 100-fold accumulation of these metabolites (Figure 2a, c). We then coupled the proton motive force-producing and -consuming processes to the conversion of lactose and oxidation of sugar, yielding reducing power in the form of the metabolic cofactor NADPH (Figure 2a, d).
NADPH provides reducing power (electrons) that is needed in numerous biosynthesis pathways. The mixing (read “coupling”) of several ingredients (biochemical modules) from L-malate decarboxylation to generation of proton motive force and forming of reducing power constitutes an important step towards the realization of self-sustaining life-like synthetic cells. Our reaction network shows a high degree of sophistication and complexity in comparison to other systems where the transport is mediated by open low-selectivity channels or pores and accumulation of metabolites is not possible. The network allows further development of out-of-equilibrium metabolism for autonomous growth of cell-like systems.
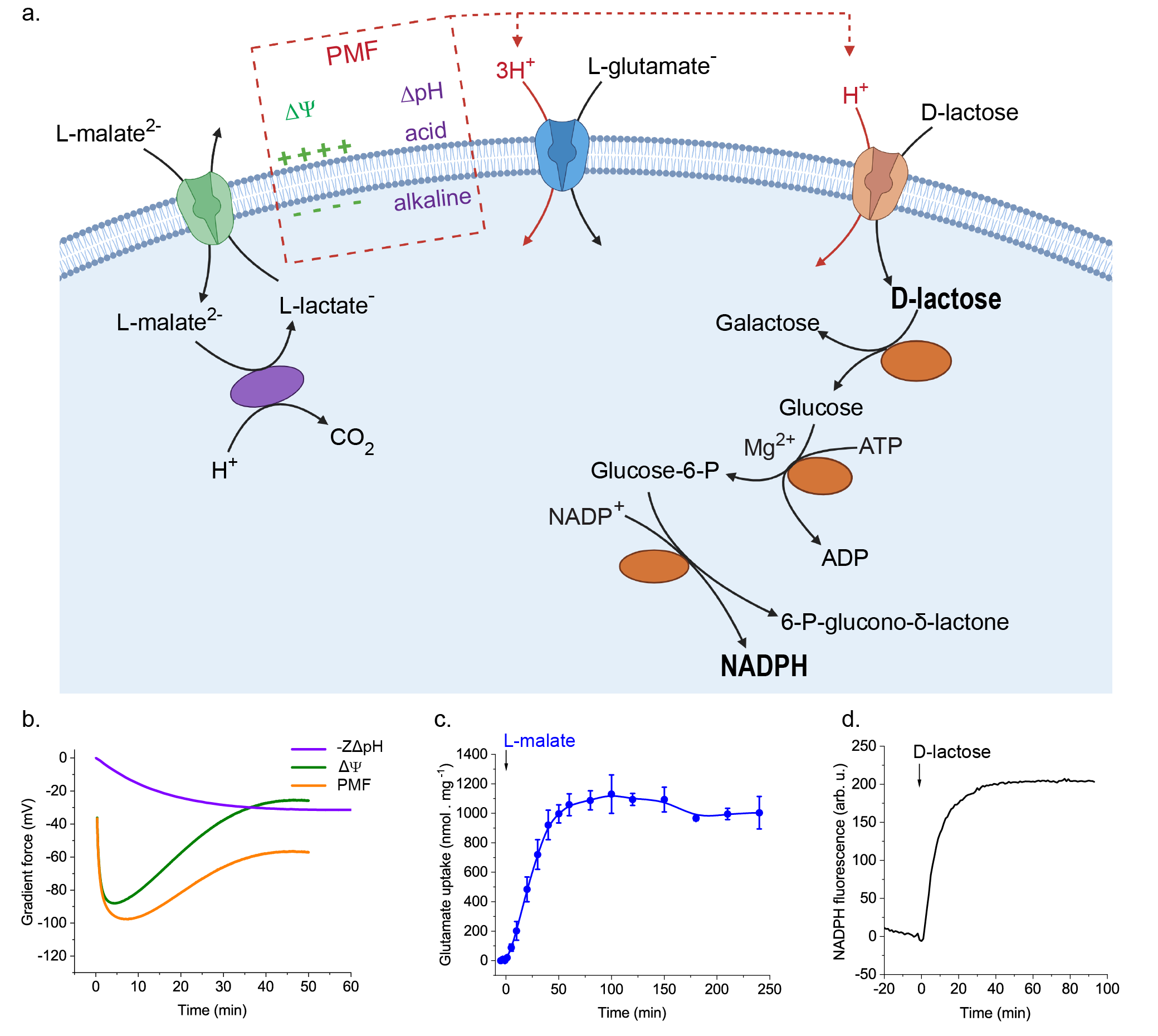
Figure 2. Transport and metabolism driven by the proton motive force from the L-malate decarboxylation pathway in lipid vesicles. a. The L-malate decarboxylation pathway reconstituted in lipid vesicles generates an electrical gradient (ΔΨ, green) and a H+ chemical gradient (ΔpH, violet), which form together the proton motive force (PMF). Co-reconstituted transporters use the stored energy from the proton motive force to drive the inward transport of L-glutamate and lactose. Internalized lactose is cleaved into galactose plus glucose; the glucose is phosphorylated and subsequently oxidized, leading to the essential NADPH. Created with BioRender.com. b. Proton motive force from the L-malate decarboxylation calculated from the measured ΔΨ and ΔpH values. c. Transport of glutamate driven by the L-malate decarboxylation. d. Metabolism of internalized lactose and production of NADPH in the here developed synthetic vesicles.
Conclusion and perspectives
Recognizing that a functioning synthetic cell must be supported by a continuous supply of metabolic energy, we show that L-malate decarboxylation constitutes a simple and effective way to generate a proton motive force in a cell-like compartment. Essential cellular functions like transport of nutrients and a minimal metabolism are driven by that proton motive force, opening the door for the incorporation of more complex functionalities and ultimately the construction of a fully autonomously functioning cell-like system.
The proton motive force obtained by the L-malate decarboxylation could be harnessed to support processes like cell division, protein secretion or communication between populations of synthetic cells. Additionally, the intermediates produced in the minimal carbohydrate breakdown pathway can serve as nodes for building a more-complex metabolic systems.
References
1 Chen, Z. et al. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat Chem Biol 14, 86-93, doi:10.1038/nchembio.2511 (2018).
2 Chen, G. et al. Implanted synthetic cells trigger tissue angiogenesis through de novo production of recombinant growth factors. Proc Natl Acad Sci U S A 119, e2207525119, doi:10.1073/pnas.2207525119 (2022).
3 Boyd, M. A. & Kamat, N. P. Designing Artificial Cells towards a New Generation of Biosensors. Trends Biotechnol 39, 927-939, doi:10.1016/j.tibtech.2020.12.002 (2021).
4 de la Fuente, M. et al. Enzyme Therapy: Current Challenges and Future Perspectives. International Journal of Molecular Sciences 22, 9181 (2021).
5 Krinsky, N. et al. Synthetic Cells Synthesize Therapeutic Proteins inside Tumors. Adv Healthc Mater 7, e1701163, doi:10.1002/adhm.201701163 (2018).
6 Miller, T. E. et al. Light-powered CO(2) fixation in a chloroplast mimic with natural and synthetic parts. Science 368, 649-654, doi:10.1126/science.aaz6802 (2020).
7 Partipilo, M. et al. Minimal Pathway for the Regeneration of Redox Cofactors. JACS Au 1, 2280-2293, doi:10.1021/jacsau.1c00406 (2021).
8 Pols, T. et al. A synthetic metabolic network for physicochemical homeostasis. Nat Commun 10, 4239, doi:10.1038/s41467-019-12287-2 (2019).
9 Bailoni, E. et al. Synthetic Vesicles for Sustainable Energy Recycling and Delivery of Building Blocks for Lipid Biosynthesis(dagger). ACS Synth Biol 13, 1549-1561, doi:10.1021/acssynbio.4c00073 (2024).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in