Engineering oat protein nanostructure to combat iron deficiency anemia
Published in Materials and Agricultural & Food Science
Iron deficiency anaemia (IDA), recognized by the WHO as the world’s most common nutritional deficiency, affects roughly two billion people, nearly a quarter of global population. Its burden falls especially heavily on young women and children: around 40% of preschool children, 30% of menstruating girls and women, and 38% of pregnant women are affected, particularly across Central and West Africa and South Asia1,2. Even mild deficiency can impact daily life, causing fatigue, headaches, dizziness, shortness of breath, and heart palpitations. Traditional treatment relies on oral iron salts such as ferrous sulfate, which is inexpensive and recommended by WHO. However, these supplements often show relatively low bioavailability and can cause undesired sensory changes, limiting their use, especially in food fortification.
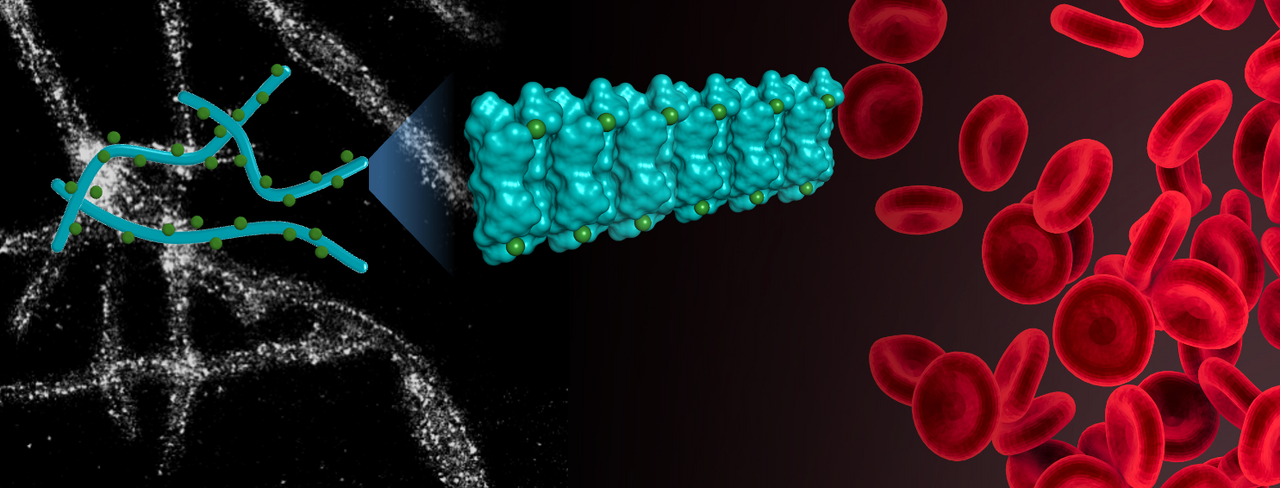
In our recent work3, we reported a plant-based strategy using oat protein nanofibrils (OatNF) carrying nm or sub-nm sized iron particles to address this problem. The idea is simple but powerful: by reducing and anchoring iron on nanostructured proteins derived from oats in presence of sodium ascorbate (OatNF-SA-Fe), we can stabilize iron in a more bioavailable form and deliver it through everyday foods and beverages. In a clinical study carried out in Thailand with 52 iron-deficient young women, our OatNF-SA-Fe compound demonstrated almost twice the absorption of ferrous sulfate, the current gold standard. Even in a polyphenol-rich food environment that typically suppresses iron absorption, this hybrid still performed remarkably better, suggesting a robust and highly effective strategy for improving iron delivery in real diets.
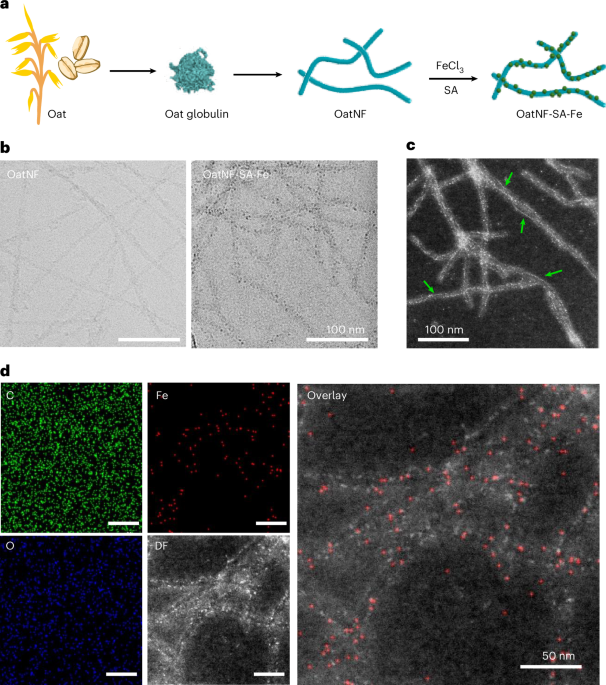
To synthesize the compound, proteins from oat powder are isolated and then allowed to self-assemble into long, thread-like nanofibrils4. These fibrils have natural binding sites that interact with metal ions. When iron salts and sodium ascorbate (SA) are introduced, ultrasmall iron nanoparticles form directly on the fibril surfaces, yielding the OatNF-SA-Fe hybrid. This approach firstly proposed by Mezzenga's team with stronger reducing agents5; here, SA provides a milder, food-safe route while the oat fibrils themselves help chelate, reduce, and stabilize iron. The final hybrid structure bears exceptionally stable ferrous iron, finely distributed along the fibrils and protected from oxidation and aggregation.
One of the key reasons this compound is absorbed so efficiently lies in how the body digests and absorb it. Our earlier in vitro and in vivo studies have shown that food protein nanofibrils break down completely in the gastrointestinal tract6. When OatNF-SA-Fe reaches the stomach, the protein scaffold is digested, and the ultrasmall iron particles dissolve rapidly due to their extremely large specific surface area. This quick release of ferrous iron in the gastrointestinal environment provides ideal conditions for absorption in the duodenum. The rational design, combining digestible protein nanofibrils with ultra-small iron particles, thus leads to fast dissolution, efficient uptake, and, ultimately, superior performance in human studies.
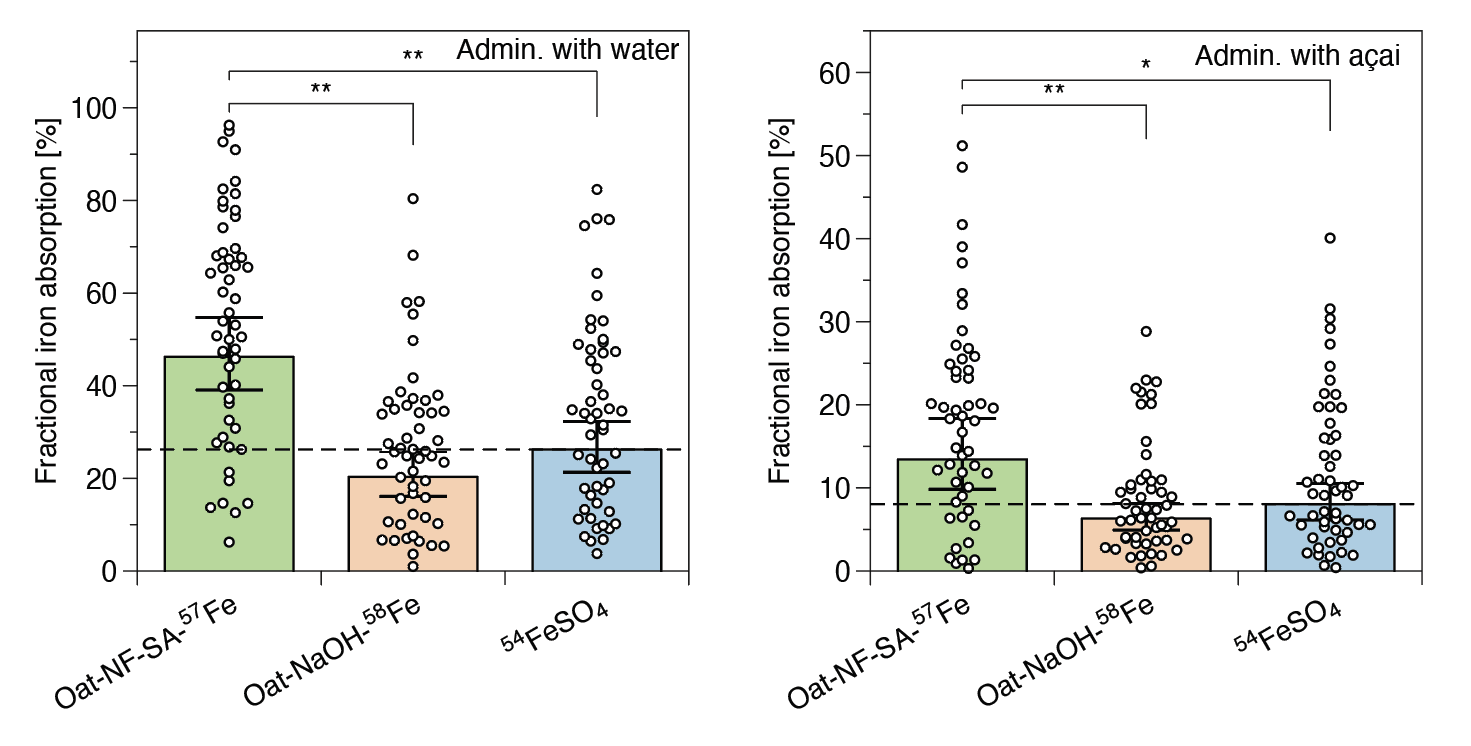
The clinical trial provides compelling evidence to validate the high bioavailability of our compound, as shown in Figure 1. Conducted with a randomized, crossover design, the study allowed each of the 52 participants to serve as her own control. When taken with water, ferrous sulfate showed absorption of about 26%, while the OatNF-SA-Fe hybrid achieved 46%, a 76% improvement. A second formulation, OatNF-NaOH-Fe, containing mostly ferric iron, still achieved around 20% absorption, demonstrating the underlying effectiveness of the nanofibril platform even when the iron state differs. When administered with a polyphenol-rich açai puree, known to reduce iron absorption, the OatNF-SA-Fe compound maintained a strong advantage, with absorption approximately 65% higher than that of ferrous sulfate. In short, the clinical data confirm that oat-based nanofibrils provide highly bioavailable iron across a range of dietary contexts.
In addition to its nutritional advantages, our plant-based formulation offers three more important benefits. First, it is particularly attractive for vegetarians and vegans, who consume only non-heme iron that found in plant foods, much less bioavailable (2–20%) than the heme iron found in meat (15–35%). Therefore, vegetarians often require nearly twice as much dietary iron to maintain healthy levels. Remarkably, our OatNF-SA-Fe hybrid, produced entirely from oat-derived ingredients, offers a rare iron supplement fully compatible with vegan diets.
Secondly, the compound demonstrates good sensory performance, an essential factor for consumer acceptance. Ferrous sulfate and some other standard compounds often cause noticeable metallic notes or color changes, but the oat-based hybrids remain largely neutral, helping ensure that fortified foods remain enjoyable, avoiding the off-flavors or visual changes that commonly limit the success of iron fortification efforts.
Finally, the long shelf life of OatNF-SA-Fe hybrid strengthens its potential global impact, especially in low-income regions where IDA is most prevalent while many iron supplements degrade during storage. In contrast, our compound retains its very high ferrous iron content even after one month of exposure to air, demonstrating exceptional stability.
Overall, the combination of high bioavailability, plant-based origin, pleasant sensory profile and durability position the OatNF-SA-Fe hybrid as a practical, affordable, and impactful option that could help address the global burden of iron deficiency.
Reference
- Lopez et al Iron deficiency anaemia. Lancet 387, 907–916 (2016). doi: 10.1016/S0140-6736(15)60865-0
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 372, 1832–1843 (2015). https://www.nejm.org/doi/full/10.1056/NEJMra1401038
- Zhou et al. Oat protein nanofibril–iron hybrids offer a stable, high-absorption iron delivery platform for iron fortification. Nature Food (2025). https://doi.org/10.1038/s43016-025-01260-6
- Zhou, J. et al. Oat plant amyloids for sustainable functional materials. Adv. Sci. 9, 2104445 (2022). https://doi.org/10.1002/advs.202104445
- Shen et al. Amyloid fibril systems reduce, stabilize and deliver bioavailable nanosized iron, Nature Nanotechnology, 12, 642–647 (2017). https://doi.org/10.1038/nnano.2017.58
- Xu et al. Food amyloid fibrils are safe nutrition ingredients based on in-vitro and in-vivo assessment, Nature Communications, 14, 6806 (2023). https://doi.org/10.1038/s41467-023-42486-x
Follow the Topic
-
Nature Food

This journal aims to provide researchers and policy-makers with a breadth of evidence and expert narratives on optimising and securing food systems for the future.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in