From Heat to Flow: A New Way to Build Graphene Pores
Published in Chemistry, Materials, and Mechanical Engineering
The idea of capturing carbon dioxide often brings to mind large industrial towers and chemical scrubbers. But imagine instead a membrane as thin as a single atom, working like a microscopic sieve that lets CO2 molecules slip through while stopping others. Such graphene membranes, with pores in ångström scale, promise to make carbon capture far more efficient and less energy intensive. Yet turning this idea into a scalable technology has long faced a major obstacle: how to reliably create these tiny, selective pores across large areas of graphene.
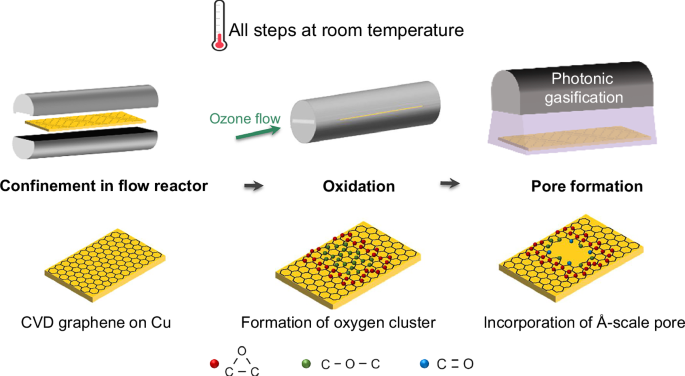
In our group, we have spent several years developing methods to create such pores in single layer graphene for gas separation applications. In our earlier work, we discovered that ozone (O3) oxidation followed by thermal gasification could introduce size selective pores that deliver attractive CO2/N2 separation. Later, we replaced the thermal step with photonic gasification, which significantly reduced the pore incorporation time. However, the oxidation process still required high temperature ozone treatment, which limited scalability and raised safety concerns. To enable industrial deployment, we need a route that worked entirely at room temperature, from oxidation to pore opening, but oxidation under these conditions had always been extremely slow.
When I began my PhD, we set out to understand why oxidation was so limited. We discovered that the reaction was not controlled by chemical kinetics, as previously assumed, but by mass transfer. In our previous study, we had shown that improving mass transfer to the graphene surface enhances oxidation efficiency. Building on this insight, we applied the same concept to achieve oxidation of graphene at room temperature, where only sluggish kinetics had been observed before.
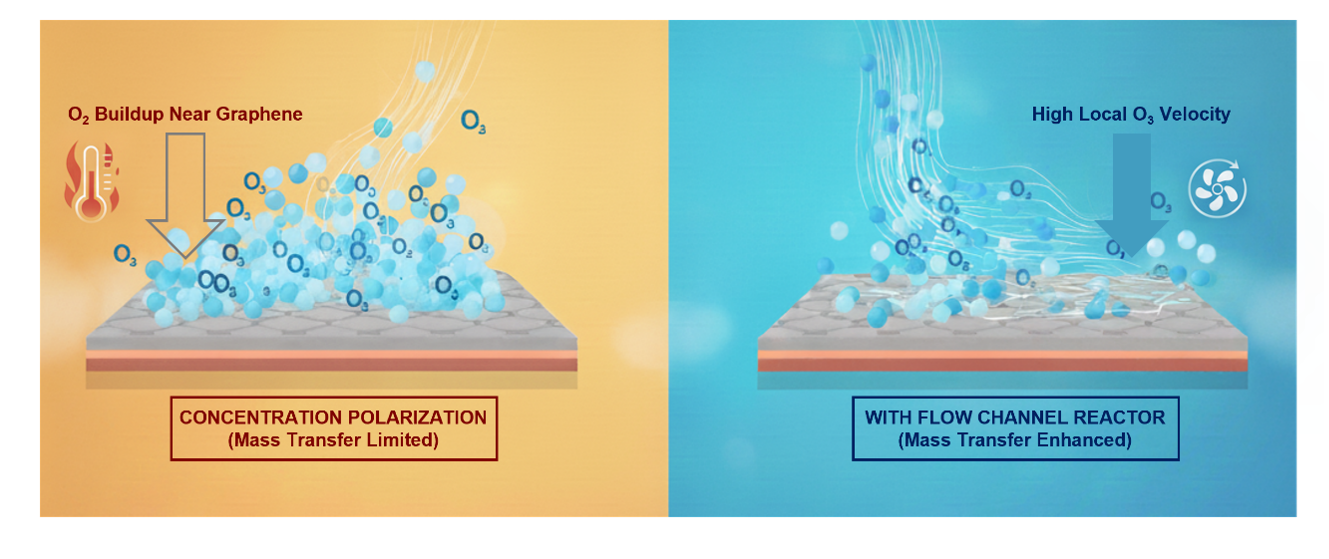
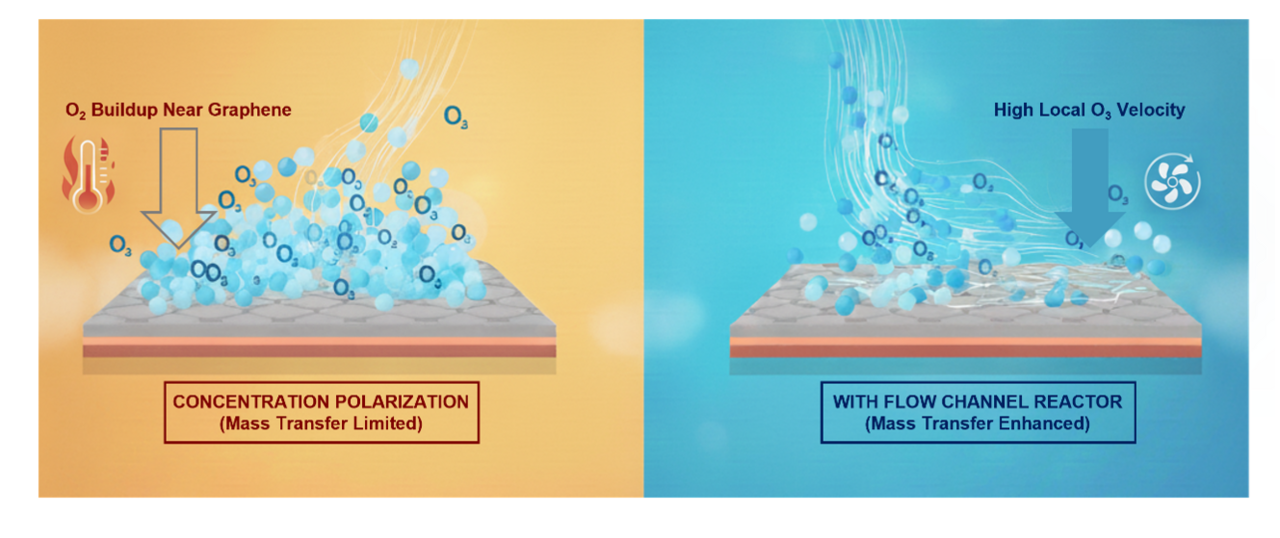
During oxidation, O3 reacts with graphene to form epoxy clusters and simultaneously produces O2 as a byproduct. The accumulation of this O2 near the surface creates a concentration polarization layer, like a traffic jam of molecules, that blocks the diffusion of fresh ozone and slows the reaction. To overcome this limitation, we redesigned the reaction environment. By introducing micro channeled slit reactors, which confine the gas in narrow gaps and significantly increase local flow velocity, we enhanced ozone transport to the graphene surface. This mass transfer enhanced oxidation accelerated the reaction by nearly an order of magnitude, enabling the formation of a high density of CO2-selective pores at room temperature.

We characterized the oxidized graphene using Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), and aberration-corrected high-resolution transmission electron microscopy (AC-HRTEM). The data consistently showed a higher degree of oxidation and an increased density of vacancy defects as flow velocity increased. AC-HRTEM imaging revealed that the room temperature process produced CO2 permeable pores with densities exceeding those obtained from high temperature oxidation. This finding demonstrated that enhanced mass transfer can fully compensate for the absence of thermal energy in driving oxidation.
We have thus established a fully room temperature route for fabricating porous graphene membranes. The centimeter scale membranes exhibit CO2 permeance exceeding 4000 GPU and CO2/N2 selectivity above 20, matching or surpassing high temperature oxidation results while eliminating the need for complex heated reactors or hazardous ozone handling.
This study builds on our group’s earlier high temperature ozone oxidation work, transforming a laboratory intensive method into a scalable and safe protocol suitable for industrial implementation. In essence, by simply rethinking how gases flow across a single atomic material we have shown that it is possible to replace heat with clever engineering of mass transfer, bringing atom thin graphene membranes one step closer to industrial reality.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Your space to connect: The Fuel cell technologies Hub
A new Communities’ space to connect, collaborate, and explore research on Electrochemistry, Chemical Engineering, and Fuel Cells!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in