From Plastics to Hydrogen: A Sustainable Catalytic Strategy
Published in Chemistry, Earth & Environment, and Materials
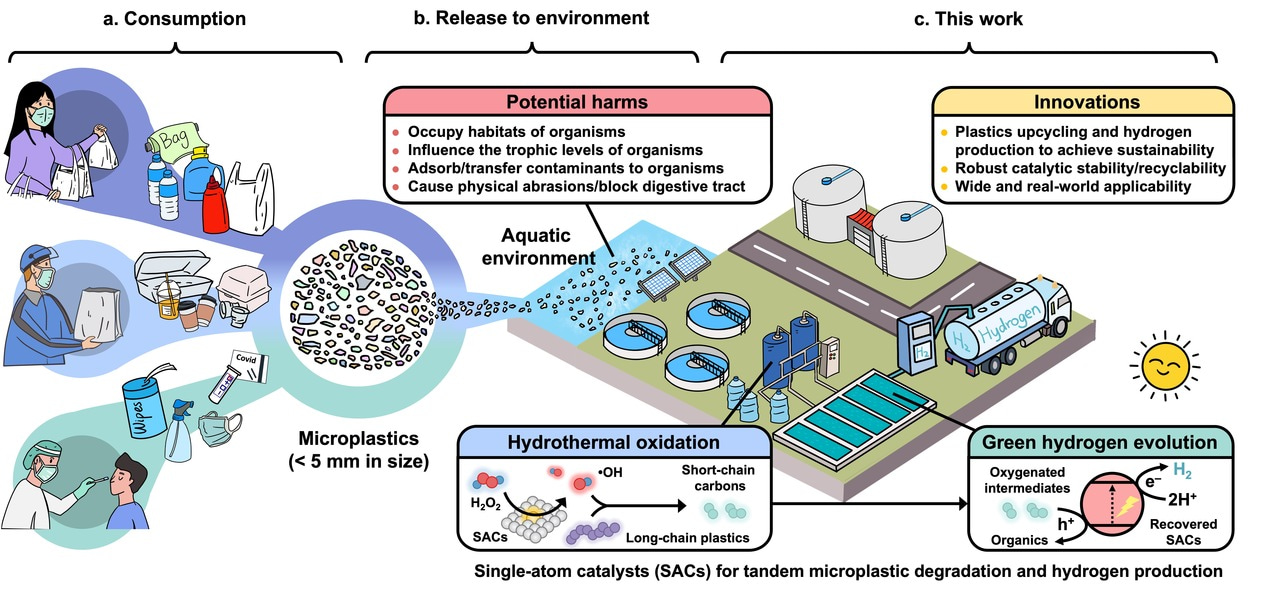
Plastic pollution has become one of the most pressing environmental challenges, as it can take hundreds of years to decompose. A staggering amount of plastic—over 400 million tons produced globally in 2022—ends up in landfills or the natural environment, where it poses risks to wildlife, marine ecosystems, and human health. Microplastics, defined as plastic particles less than 5 mm in size, represent a particularly insidious facet of this pollution. They originate from a variety of sources, including the breakdown of larger plastic items, synthetic textiles, and even personal care products. Once released into the environment, microplastics are nearly impossible to retrieve, leading to their widespread distribution in oceans, rivers, soil, and air. The ecological implications of microplastics are profound. Marine organisms, from plankton to larger fish, can mistake these particles for food, leading to ingestion that can cause physical harm and toxic exposure. These microplastics can enter the food chain, potentially impacting human health.
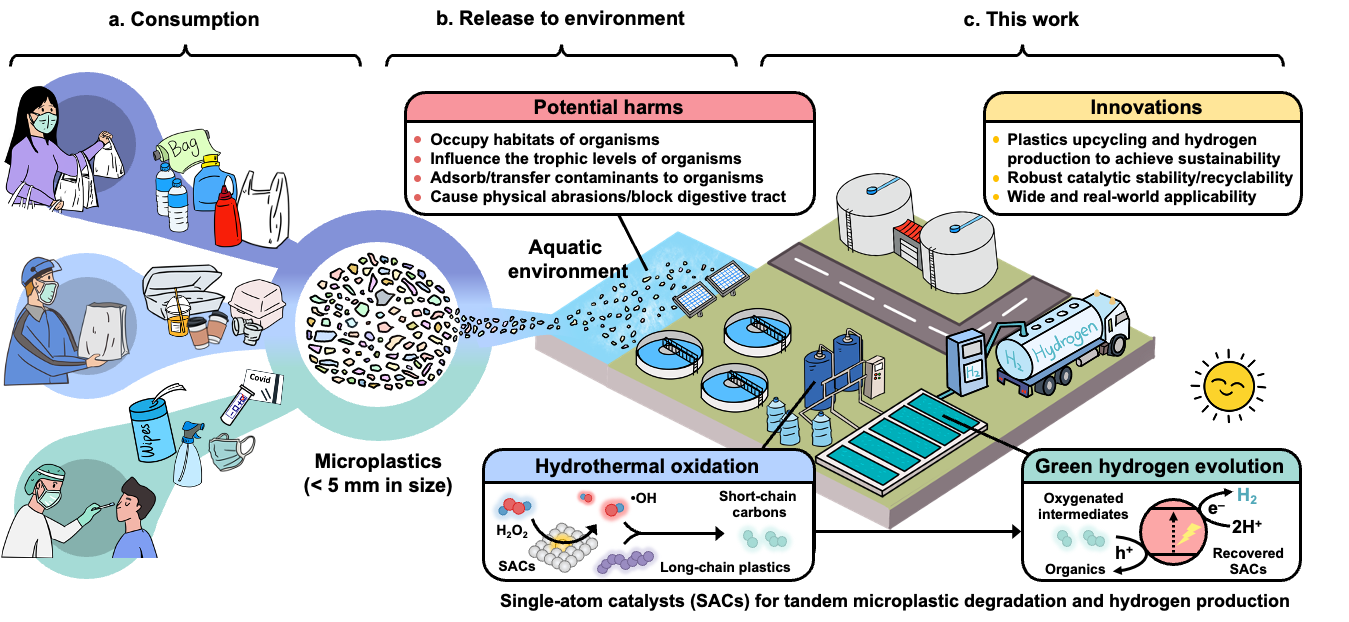
Figure. Plastic consumption, release, and new strategy of microplastics upcycling. a Consumption and generation of microplastic wastes. b Release and potential harms of microplastic wastes. c, Proposed strategy of a tandem microplastic degradation-hydrogen evolution reaction (MPD-HER) process.
Efforts to address plastic pollution include enhancing recycling processes, developing biodegradable alternatives, and implementing stricter regulations on plastic production and use. Public awareness campaigns also play a crucial role in reducing plastic consumption and encouraging more sustainable practices. However, tackling the issue of microplastics requires innovative solutions, such as advanced filtration systems, cleanup initiatives, and research into effective methods for removing these particles from the environment. Recent research has explored photocatalysis, electrocatalysis, and photoelectrochemistry to break down microplastics while generating hydrogen. However, these methods often require strong acids or bases, leading to environmental hazards, safety risks, and high costs. They also only work on a few types of plastics. Fenton and Fenton-like systems, which use oxidants like hydrogen peroxide to produce reactive oxygen species, show promise for degrading a wider range of microplastics. Yet, these methods face challenges like low efficiency, poor catalyst recyclability, harsh acidic conditions, and waste generation. Therefore, more sustainable and efficient solutions are needed to tackle microplastics pollution.
In our study, we developed a novel tandem catalytic process, which effectively integrates the degradation of microplastics with photocatalytic green hydrogen production. Central to this process is a hierarchical carbon nitride-supported single-atom iron catalyst (FeSA-hCN). The FeSA-hCN catalyst with Fe with Fe−N4 sites can effectively activate hydrogen peroxide (H2O2) to generate strong hydroxyl radicals (•OH). These radicals can facilitate the breakdown of ultrahigh molecular-weight polyethylene (UHMWPE) by braking the C−C bond and introducing oxygen-ralted groups into the carbon chain. Under hydrothermal working conditions, nearly complete degradation of UHMWPE was achieved. This system also demonstrates remarkable stability across six cycles (a total of 72 hours). Our Fenton-like system excels in terms of microplastics degradation efficiency and catalyst recyablity, outperforming many existing solutions documented in the literature.
More importantly, this method exhibited excellent real-life applicability in oxidizing microplastics in different natural water bodies, including tap, lake, river, and seawater. We further investigated the degradation of different polymer types, including polyethylene terephthalate (PET), high-density polyethylene (HDPE), polyvinyl chloride (PVC), low-density polyethylene (LDPE), polypropylene (PP) and polystyrene (PS). Our system shows a wide applicability in degrading most types of plastic products. Further extending to real-life plastics, our method also exhibited great effectiveness in degrading different everyday plastic products, including plastic bags, ‘hand and surface wet wipes’ plastic bottles, plastic food containers, plastic drink bottles, and medical ziplock bags. The high activity, stability, and applicability of this system under real-world conditions make it highly promising for practical use, offering great potential in tackling real-life plastic pollution problems.
Even more exciting is that the system not only breaks down plastics but also converts the degradation products into valuable carboxylic acids, which play a role in driving a subsequent reaction for hydrogen production. This tandem system not only outperforms other plastic-degrading technologies but also exceeds most hydrogen-producing methods in terms of efficiency. By using the recovered FeSA-hCN as photocatalysts and UHMWPE-derived products as sacrificial agent, a hydrogen production rate of 42 µmol h−1 was achived under light irradiation. This process offers a practical way to recycle plastic while simultaneously generating fuel for a cleaner energy future.
The implications of this research are profound. Imagine a future where plastic waste from homes, businesses, and even oceans can be collected and converted into a source of clean energy. This would not only reduce the amount of plastic in the environment but also help meet the world’s growing energy needs in a sustainable way. While this breakthrough is still in the early stages, the potential for real-world applications is enormous. Our team is now focusing on improving the process, expanding the range of plastics that can be broken down, and making the technology more efficient. By transforming plastic waste into green energy, this research addresses a critical environmental challenge while paving the way for a cleaner, more sustainable future.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Your space to connect: The Fuel cell technologies Hub
A new Communities’ space to connect, collaborate, and explore research on Electrochemistry, Chemical Engineering, and Fuel Cells!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in