Groundwater arsenic removal by a bioinspired porous membrane
Published in Earth & Environment
The International Agency for Research on Cancer (IARC) has classified As as a class 1 carcinogen and the World Health Organization (WHO) set a guideline value of 10 ppb as its limit for drinking water, which has been adopted by most state governments as regulatory limit. Several conventional and emerging removal technologies have been implemented so far for arsenic removal from water, which include oxidation, coagulation flocculation, adsorption, ion exchange, and membrane separation processes. However, these methods suffer from different drawbacks, such as high waste production, low efficiency, high environmental impact, and high operative costs, which may make them not easily accessible in large scale. Regarding membrane processes, in particular, nanofiltration (NF), forward osmosis (FO), reverse osmosis (RO) and membrane distillation (MD) are important technologies, able to remove up to 100% of As by a filtration process, but they also cause the removal of the major part of or even all the other dissolved minerals present in water, making it unsuitable for direct human consumption. Moreover, current membrane techniques are characterized by a limited efficiency in the removal of As(III), which, on the other hand, is the most toxic As species, so pretreatment of water with suitable oxidants for favoring the oxidation of As(III) to As(V) is usually needed. We have now developed a novel porous water-permeable porous membrane, which has been made selective toward the removal of arsenic in both its oxidation states (III and V) by chemically incorporating into the membrane matrix an imidazolium-based ionic liquid functionalized with a thioester group (PIL-S), with high and specific affinity for arsenic. The porous nature of the PIL-S‒functionalized membrane (PIL-S-M) allows a high water percolation, while the membrane selectively removes As(III) as well as As(V) with high efficiency, independently from the initial As concentration. Thus, after a single operation, a filtrated water with an As concentration below the WHO guideline is obtained. Since only As is selectively retained, while other metals, metalloids, ions, etc. can easily permeate the membrane, the permeate maintains a mineral content similar to original water, so no significant demineralization occurs (contrary to NF, FO, RO, or MD techniques where the filtered water needs to be added of salts to make it suitable for drinking or other uses).
The new bioinspired PIL-S monomer (namely, 3-(6-(acetylthio)hexyl)-2-methyl-1-(4-vinylbenzyl)-1H-imidazol-3-ium bromide) has been specifically designed for conferring to the membrane PIL-S-M, obtained upon its copolymerization with other acrylate monomers, high affinity for interaction with arsenic in both its oxidation states (As(III) and As(V)), as shown in Fig. 1a. It is in fact known that that binding of As to proteins takes place with two sulfur atoms (from sulfhydryl groups) in the protein active site, and in our case the presence of the thioester group in PIL-S would favor the formation of strong covalent bonds between the arsenic center and two sulfur atoms of the copolymerized PIL-S. Further stabilization could also be provided by the interaction between the imidazolium cation moieties and the oxygen atom(s) bonded to arsenic, as shown in Fig. 1b and 1c. This hypothesis has been corroborated by ab-initio calculations, performed using a simplified model system consisting of a fragment (single monomer) of the polymeric material barebone, and then experimentally confirmed by EXAFS (extended X-ray absorption fine structure) synchrotron experiments and XPS (X-ray photoelectron spectroscopy) analysis of the membrane after filtration of model As(III) solutions.
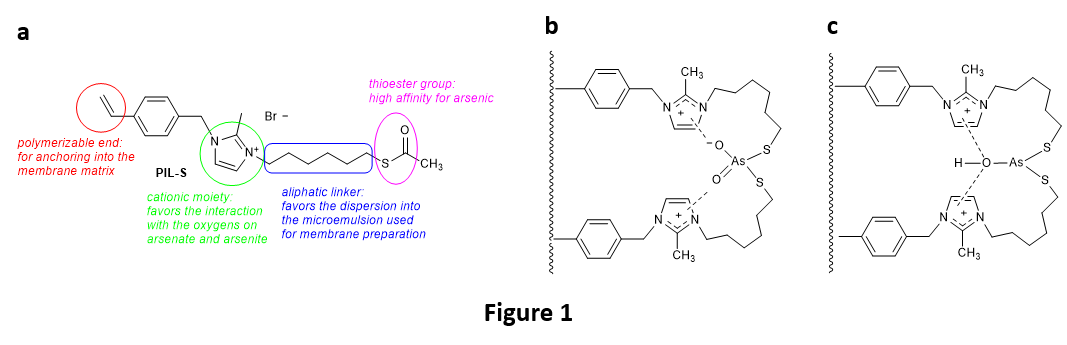
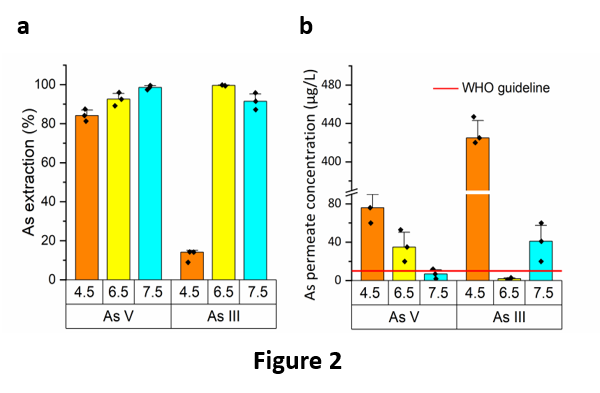
The PIL-S monomer has been chemically synthesized and the new porous membrane PIL-S-M easily produced upon polymerization of a microemulsion made of water, PIL-S-M, methyl methacrylate (MMA), 2-hydroxyethyl methacrylate (HEMA), ethylene glycol dimethacrylate (EGDMA), polyethylene glycol (PEG-400), and a surfactant (dodecyltrimethylammonium bromide, DTAB). It has showed excellent performances in the removal of both As(V) and As(III) from model solutions, using a dead-end filtration apparatus. In particular, the best results were obtained at pH values of 7.5 and 6.5 for As(V) and As (III), respectively (Fig. 2a and 2b). The extraction efficiency of the membrane was up to 99.6 % (Fig. 2a), thus abating the concentration of As in the permeate below the WHO guideline (10 μg/L). Of particular importance is the high extraction capacity of the membrane towards As(III), which is the most toxic species of As and the most recalcitrant to be extracted, so much that its removal often requires a pre-oxidation step.
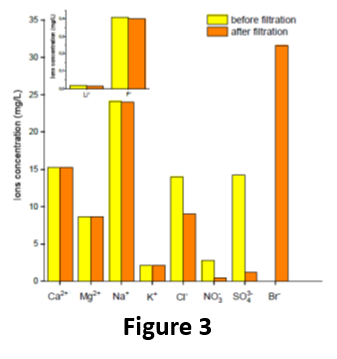
 Even more importantly, the membrane was effective in selectively removing arsenic from a naturally contaminated groundwater, in particular, a real groundwater called “Sorbo 2”, containing ca. 65 μg/L of arsenic (90% As(V) and 10% As/(III)) and collected from Calabrian mountains (Sila Massif, Calabria, Italy). After a single operation, the membrane was able to retain ca. 85 % of As, thus allowing to decrease the As concentration in the filtrated water to less than 10 μg/L. Moreover, the performance of PIL-S-M was almost unaffected by the presence of the other ions present in the groundwater. In fact, the concentration of the main ions before and after the filtration trough PIL-S-M remained almost unchanged (Fig. 3). This means that our porous PIL-S-M was able to sequester As without affecting the other ionic species (such as Li+, Ca2+, Na+, K+, and F‒), which freely permeated through the pores of the membrane. This result was outstanding, considering that in one single passage through a porous membrane it was possible to abate the concentration of As at the safety standard suggested by the WHO, without causing significant water demineralization, which therefore becomes directly usable for human consumption.
Even more importantly, the membrane was effective in selectively removing arsenic from a naturally contaminated groundwater, in particular, a real groundwater called “Sorbo 2”, containing ca. 65 μg/L of arsenic (90% As(V) and 10% As/(III)) and collected from Calabrian mountains (Sila Massif, Calabria, Italy). After a single operation, the membrane was able to retain ca. 85 % of As, thus allowing to decrease the As concentration in the filtrated water to less than 10 μg/L. Moreover, the performance of PIL-S-M was almost unaffected by the presence of the other ions present in the groundwater. In fact, the concentration of the main ions before and after the filtration trough PIL-S-M remained almost unchanged (Fig. 3). This means that our porous PIL-S-M was able to sequester As without affecting the other ionic species (such as Li+, Ca2+, Na+, K+, and F‒), which freely permeated through the pores of the membrane. This result was outstanding, considering that in one single passage through a porous membrane it was possible to abate the concentration of As at the safety standard suggested by the WHO, without causing significant water demineralization, which therefore becomes directly usable for human consumption.
Follow the Topic
-
Nature Water

This journal publishes research on the evolving relationship between society and water resources on a monthly basis. It covers the natural sciences, engineering, and social sciences, with a particular interest in regards to interdisciplinary research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Remote sensing and forests
Publishing Model: Hybrid
Deadline: Apr 30, 2026
Water pollution and advanced treatment processes
Publishing Model: Hybrid
Deadline: Feb 28, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in