
Dr C. Gilles’s interest in Epithelial-Mesenchymal Transitions (EMTs) goes back in the early 90s when EMT was mostly associated to migration/invasion mechanisms. Today, EMTs have been shown to play crucial roles in the biology of circulating tumor cells (CTCs) derived from epithelial tumors and to provide tumor cells with increased invasive, survival, stemness and niching properties (1). It is nowadays recognized that EMT-shifted CTCs encompass pro-metastatic subpopulations. Nevertheless, the mechanisms underlying metastatic colonization by EMT-shifted CTCs remain underexplored.
Vimentin is certainly amongst the best known marker of EMT. Vimentin is a type III intermediate filament, constitutively expressed in mesenchymal cells such as fibroblasts or endothelial cells for instance, playing key roles in EMTs, notably by its involvement in phenotypic changes during this process (2,3).
Recently, the group identified an EMT-driven axis leading to the overexpression of Tissue Factor (TF), and providing tumor cells with coagulant properties that facilitate early metastatic colonization of CTCs in experimental metastasis mouse assays (4). Tissue Factor is indeed a cell-membrane associated activator of the coagulation cascade. Based on this previous work emphasizing a narrow association between vimentin and TF expression in vitro, in human breast cancers and in CTCs isolated from metastatic breast cancer patients, we examined here the possibility that the canonical EMT marker vimentin could directly contribute to TF regulation, and thereby to early metastasis.
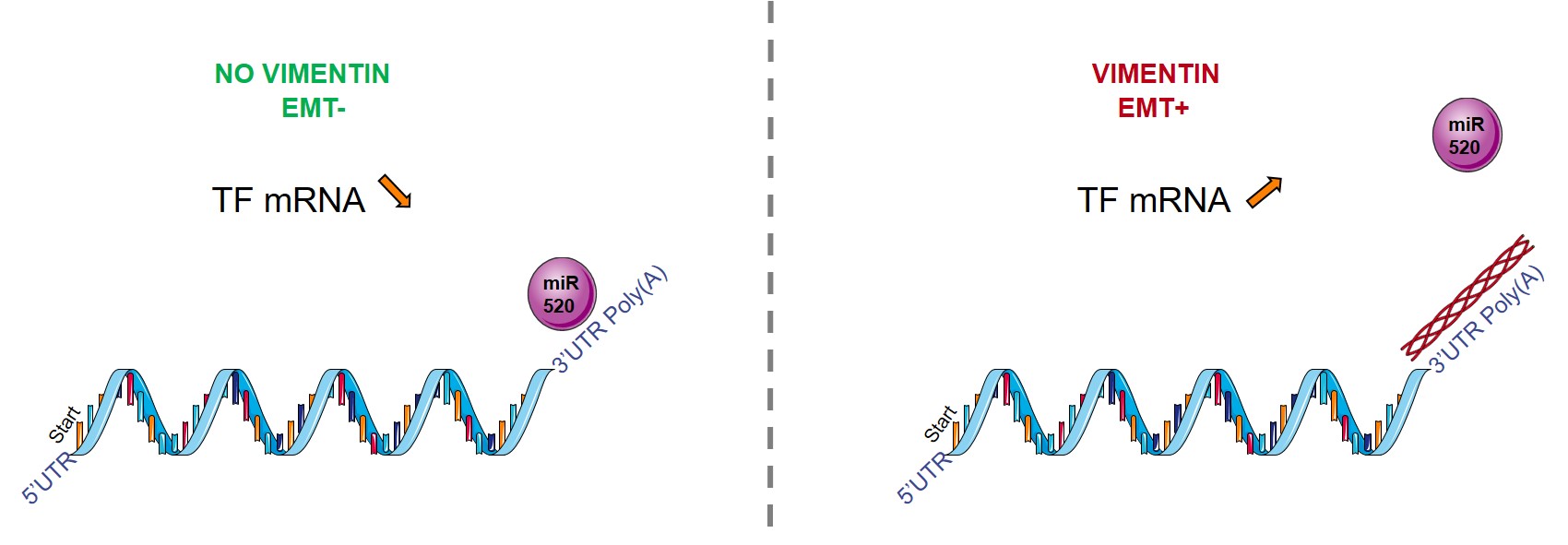
In this paper, we thus show in several EMT cellular systems, vimentin silencing diminishes TF expression, coagulant activity and early metastasis. Digging further the molecular mechanism underlying these observations, we identified a novel regulatory mechanism of TF by vimentin. Different evidence indeed point to a post-transcriptional regulatory mechanism of TF mRNA by vimentin: (i) vimentin silencing accelerates TF mRNA decay after actinomycin-D treatment, reflecting TF mRNA stabilization, (ii) RNA immunoprecipitation reveals enriched levels of TF mRNA in vimentin immunoprecipitate, (iii) TF 3’-UTR-luciferase reporter vector assays implicated the 3’-UTR of TF mRNA in vimentin-dependent TF regulation, and (iv) using different TF mRNA 3’UTR luciferase reporter vectors mutated for potential miR binding sites and specific Target Site Blockers identified a key miR binding site in vimentin-dependent TF mRNA regulation.
All together, these data support a novel mechanism by which vimentin interferes with a miR-dependent negative regulation of TF mRNA, thereby promoting coagulant activity and early metastasis of vimentin-expressing CTCs.
Written by Aline M. Vanwynsberghe & Dr. Christine Gilles
1. Francart ME, Lambert J, Vanwynsberghe AM, Thompson EW, Bourcy M, Polette M, et al. Epithelial-Mesenchymal Plasticity and Circulating Tumor Cells: Travel Companions to Metastases. Dev Dyn 2017
2. Lowery J, Kuczmarski ER, Herrmann H, Goldman RD. Intermediate Filaments Play a Pivotal Role in Regulating Cell Architecture and Function. J Biol Chem 2015;290:17145-53
3. Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 2008;68:989-97
4. Bourcy M, Suarez-Carmona M, Lambert J, Francart ME, Schroeder H, Delierneux C, et al. Tissue Factor Induced by Epithelial-Mesenchymal Transition Triggers a Procoagulant State That Drives Metastasis of Circulating Tumor Cells. Cancer Res 2016;76:4270-82
Follow the Topic
-
Oncogenesis

A peer-reviewed open access online journal that publishes articles exploring mechanistic insight and molecular basis of cancer and related phenomena. It seeks to promote diverse and integrated areas of molecular biology, cell biology, oncology, and genetics.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in