How can stress affect our children?
Published in Neuroscience, Cell & Molecular Biology, and Genetics & Genomics
We live in an increasingly stressful world. We know that chronic stress can compromise our health drastically, as shown by the increasing reports of depression, burnout, and anxiety disorders. What is less known however is whether our stressful lifestyles can have an impact on the health of our children. The impact of our genetics determining our children’s health is well established, and more and more studies describe a correlation between our lifestyles and offspring health. How can this happen? Our lifestyle choices and our environment might influence the epigenome of our germ cells. The altered epigenetic state of our germ cells subsequently might influence the developmental trajectory of the unborn children. The exact mechanism for this form of non-genetic inheritance is not yet fully understood.
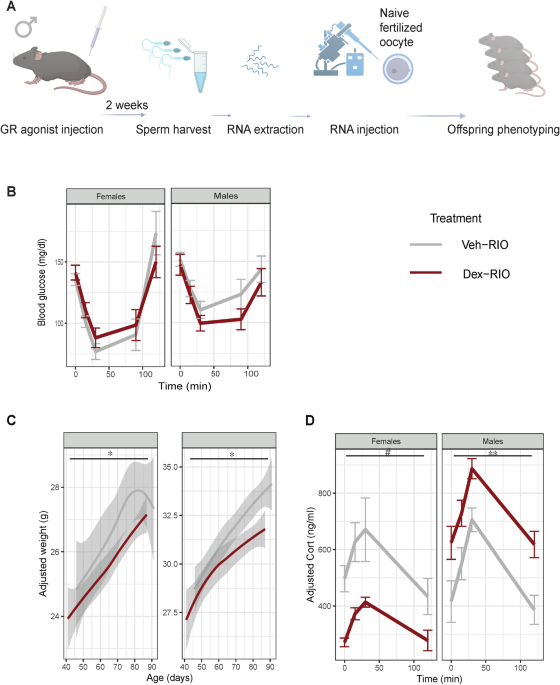
While the majority of efforts in the field went into describing non-coding RNAs as such a mechanistic link, we wanted to explore other potential candidates. This was rooted in the observation that offspring resulting from injection of RNA harvested from sperm of stress exposed male mice into fertilized oocytes did not fully recapitulate the aberrant phenotype of offspring born from in vitro fertilization using stressed father´s sperm. Exploring alternative epigenetic modifications in sperm, we observed changes in chromatin accessibility of fathers exposed to a pharmacological stress mimic and stress hormone agonist, Dexamethasone (Dex). Among others, we observed decreased chromatin accessibility in a region encoding the protein Sos2, which regulates insulin signalling. As offspring resulting from Dex exposed fathers displayed a metabolic phenotype, we investigated whether Sos2 gene expression in said offspring could be affected by altered accessibility in paternal sperm. Indeed, we could observe a downregulation of Sos2 in the offspring´s liver, which was not present in the liver of offspring generated from in vitro fertilization.
While this example demonstrated a link between stress induced chromatin accessibility changes in exposed father´s sperm and one observable offspring phenotype, we wanted to investigate this on a global level as well. Dex activates the main stress hormone, the glucocorticoid receptor (GR), which on a cellular level acts as a transcription factor that can modify chromatin states. In sperm of Dex exposed males, accessibility in regions with the motif archetype of the transcription factor family including the motif for GR was decreased. More specifically, activity of the motif for GR and the progesterone receptor was significantly changed at sites marked by 5-Formylcytosine (5fC), which is an intermediate of the DNA methylation cycle produced by the ten-eleven translocation enzymes.
We wondered whether this 5fC mark might be functionally relevant in regards to chromatin remodelling upon Dex treatment. For this, we employed a heterozygous knockout mouse line, lacking thymine-DNA glycolase (TDG). In accessible sites of heterozygous Tdg knockout animals, we observed a higher activity of the motif for GR. A very similar motif, the androgen receptor, was also affected the most in mouse embryonic stem cells with the heterozygous knockout. Given that there are no androgen receptor transcripts in embryonic stem cell expression data, this could indicate that GR is activating genes that in other cell types would by controlled by the androgen receptor.
Taken together, our results suggest a relationship between TDG, steroid receptors and chromatin accessibility in general and further substantiate a mechanistic contribution to the intergenerational effects of Dex exposure.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Related Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Jun 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in