How the first brain (the brain in the gut) controls gut movements
Published in Healthcare & Nursing

The gastrointestinal (GI) tract is the only internal organ to have evolved over hundreds of millions of years with its own nervous system, called the Enteric Nervous System (ENS). Activity from the ENS alone is sufficient to generate complex motor patterns that propel content along the GI-tract. Animals like Hydra, have been around for over 600 million years with an intrinsic nervous system, but no brain or spinal cord, as we know it. Hydra still exist today and despite the lack of brain or spinal cord, demonstrate complex movements (like peristalsis and propulsion of ingested content), without conscious thought. They only use their intrinsic nervous system. Hence, we suggest the brain in the gut is the first brain, because it clearly evolved before the complex brain, that we have evolved to use much more recently every day to control our voluntary movements, such as walking and talking.
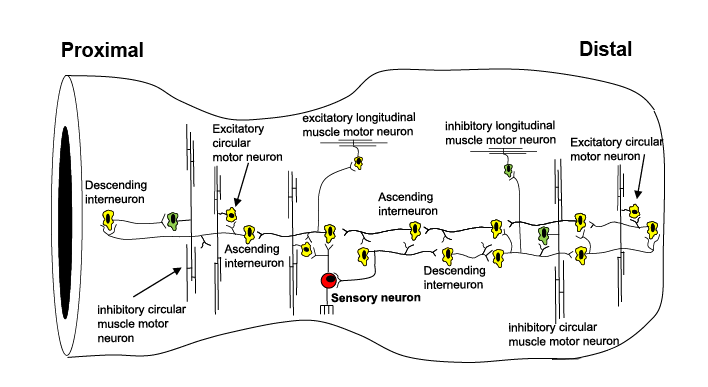
The ENS in the gut consists of many hundreds of thousands of neurons that are contained in small ganglia that form an interconnected network (the largest called the myenteric plexus), where each ganglion consists of a heterogenous population of neurons. This means any individual ganglion (within the ENS) contains a mixed population of neurons, which could be an intrinsic sensory neuron, an interneuron, or excitatory or inhibitory motor neuron.
One of the most challenging questions that has faced enteric neuroscientists is how all the different classes of neurons are temporally and spatially activated to generate complex patterns of motor activity, that underlie propulsion along the gut. In this study, we provide a major new insight into how all the different neurochemical classes of myenteric neurons are temporally and spatially activated along the colon, to generate propulsive contractions (Spencer, 2021). Interestingly, the same neural circuit was activated during both propulsive and non-propulsive contractions. To perform this study, we took advantage of a recent technical advance from our laboratory (Costa et al., 2019) that enables smooth muscle electrical recordings along the length of colon to be correlated with dynamic changes in colonic wall diameter during propulsion. This allows the patterns of neuronal activity in the ENS that underlie propulsion to be inferred (Spencer et al., 2018). We reveal a mechanism that explains how ENS activity underlies propulsion of content along the colon.
The findings in this study show that coordinated firing of many thousands of ascending and descending interneurons synaptically activate large populations of excitatory and inhibitory motor neurons, not only orally behind the bolus, but also over considerable distances downstream, ahead of the propagating contraction wavefront. This was a significant step forward because it means the temporal delay in onset of smooth muscle contraction ahead of a propagating contraction is due to the preferential aboral projections of inhibitory motor neurons, which, when active, suppress the smooth muscle excitation by the concurrently active excitatory motor neurons (Fig.1). The electrophysiological recordings made concurrently with the spatio-temporal D-mapping showed pulsatile firing of excitatory and inhibitory neuromuscular inputs not only in proximal colon, but also distal colon, long before the propagating contraction invades the distal region (Fig.1). During the propulsion of fluid along the isolated colon, wavelet analysis showed there was increased coherence of excitatory junction potentials occurring at ~2Hz over large distances, between the proximal and distal regions (Fig.1). Therefore, during propulsion, synchronous firing of descending inhibitory nerve pathways over long ranges aborally acts to suppress the smooth muscle from contracting, counteracting the excitatory nerve pathways active over this same region of colon (Spencer, 2021).
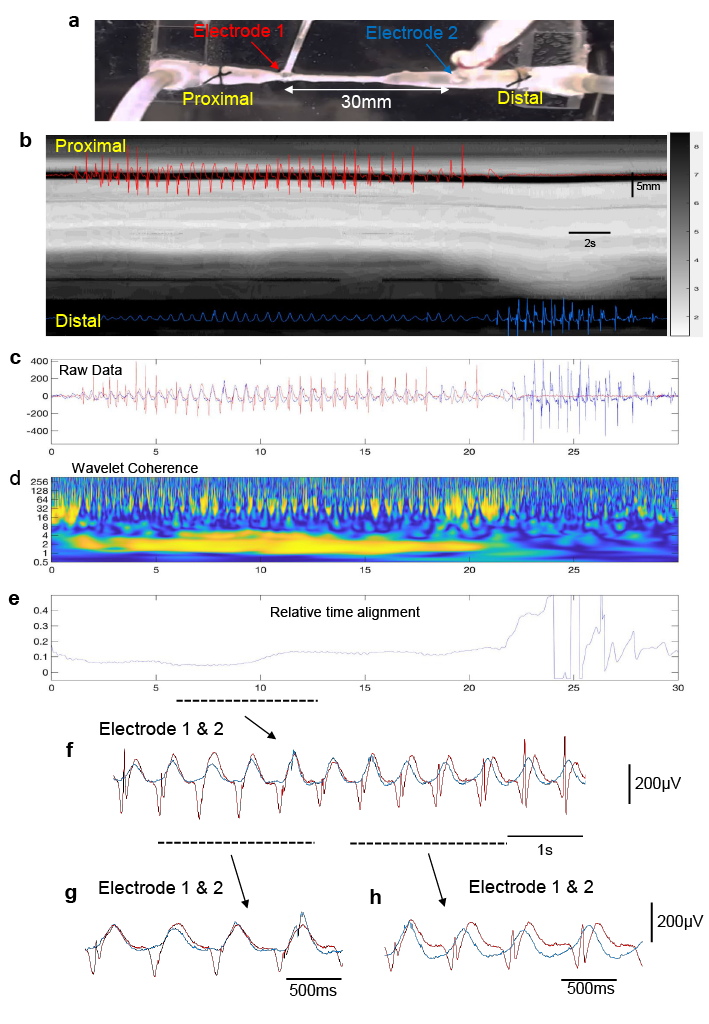
As mentioned above, from an evolutionary perspective the ENS is the first brain, as it evolved before the CNS (Furness and Stebbing, 2018, Spencer et al., 2018, Spencer and Hu, 2020). The ENS represents a primeval hard-wired neural control system, which, when active is essential for neurogenic propulsion along the colon. Our work reveals a unique feature of the ENS of the colon that underlies both non-propulsive and propulsive motor patterns. The study demonstrates the existence of large functional assemblies of neurons in the ENS that can self-organise to generate coordinated firing considerable distances along the colon (Fig.2). This activity underlies the basis of an important motor pattern in the colon. The findings uncovered here are far more complex than we expected and considerably different from the mechanisms that underlie the propulsion of fluid along other hollow smooth muscle organs that have evolved without an intrinsic nervous system; like in lymphatic vessels, ureters or the portal vein. Synchronization of neuronal activity across large populations of neurons is common in the nervous system of many vertebrate animals. This study shows that the ENS behaves similar to other complex neural networks in the brain and spinal cord of other species.
REFERENCES:
COSTA, M., KEIGHTLEY, L. J., WIKLENDT, L., HIBBERD, T. J., ARKWRIGHT, J. W., OMARI, T., WATTCHOW, D. A., ZAGORODNYUK, V., BROOKES, S. J. H., DINNING, P. G. & SPENCER, N. J. 2019. Roles of three distinct neurogenic motor patterns during pellet propulsion in guinea pig distal colon. J Physiol. 597(20):5125-5140.
FURNESS, J. B. & STEBBING, M. J. 2018. The first brain: Species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol Motil,. Feb;30(2). doi: 10.1111/nmo.13234.
SPENCER, N. J., HIBBERD, T. J., TRAVIS, L., WIKLENDT, L., COSTA, M., HU, H., BROOKES, S. J., WATTCHOW, D. A., DINNING, P. G., KEATING, D. J. & SORENSEN, J. 2018. Identification of a Rhythmic Firing Pattern in the Enteric Nervous System That Generates Rhythmic Electrical Activity in Smooth Muscle. J Neurosci, 38, 5507-5522.
SPENCER, N. J. & HU, H. 2020. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol. 17(6):338-351.
SPENCER, N. J., TRAVIS, L, WIKLENDT, L, HIBBERD, T.J, COSTA, M, BROOKES, S.J, DINNING, P.G, HU, H, WATTCHOW, D.W, SORENSEN, J. 2021. Long Range Synchronization within the Enteric Nervous System Underlies Propulsion Along the Large Intestine of Mice. Communications Biology In press.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Welcome