How ticks stick: on the phase separation and ageing of glycine-rich protein from tick adhesive

When I look back at how this entire story about the potential role of intrinsically disordered proteins (IDPs) in tick adhesion started, I cannot help but feel a sense of extreme satisfaction on how a curiosity-driven exploration can lead to an in-depth study and give insights into an unexplored biological question. To begin with, I never intended to study tick adhesion as such: being fascinated by IDPs and their ability to undergo phase separation and form membraneless condensate droplets, I simply stumbled upon this peculiar protein family called GRP during one of my internet searches. What was exciting was that these proteins were found in tick saliva – a remarkable proteinaceous mixture that ultimately transforms into a strong bioadhesive called a cement cone (Figure 1a). But one look at the sequence, and I was convinced that GRPs should exhibit some sort of phase separation. What followed next was an exciting three-year journey that exposed me and my lab to a fascinating biological question, and a chance to shape a truly interdisciplinary project in a highly collaborative manner.
All you need is enthusiasm… and some (wo)manpower!
I immediately started to look for tick researchers in the Netherlands. There were not so many. There was one group at Wageningen University, but this group focusses more on the ecology and not so much on protein biochemistry; funnily enough, we ended up collaborating with them later on in the project to perform preliminary experiments with natural tick saliva. However, one name stood out: Ingrid Dijkgraaf from Maastricht University. Sometimes, things are just meant to be, because when I emailed Ingrid about my out-of-the-blue hypothesis about GRPs undergoing phase separation and somehow being linked to tick adhesion, I got an immediate reply from her saying that they were in fact in the process of synthesizing a GRP for another completely different reason! Meanwhile, I pitched this project to an enthusiastic Master’s student, Polina, who immediately signed up for the task, fully knowing the risk of diving into a completely new project.
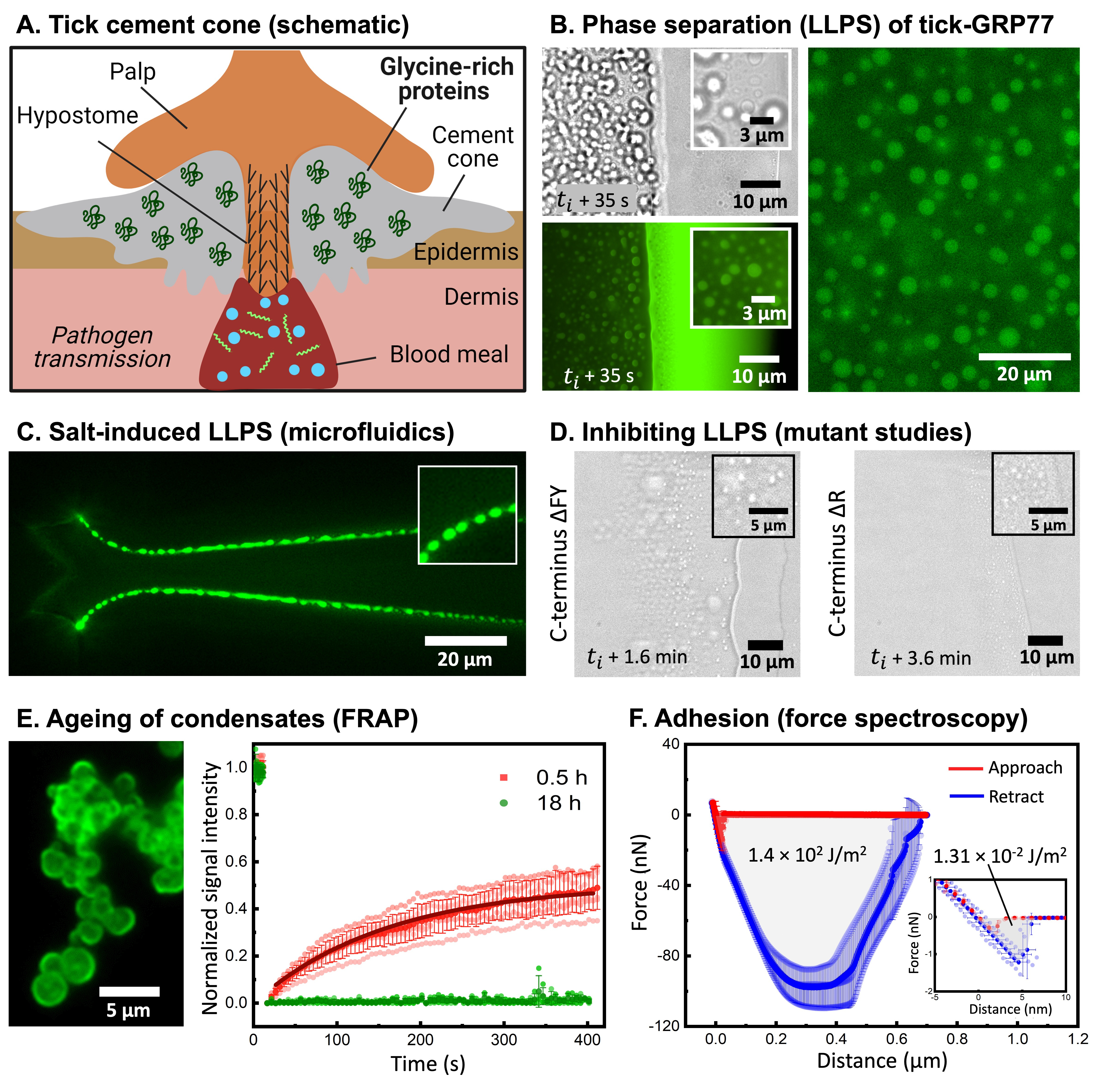
Soon, a small amount (less than half a milligram) of GRP was posted to us by Ingrid, but under the capable hands of Polina and Ketan (my first PhD student, who was happy to take this up as a side-project), we could use it for well over a year and did numerous experiments. With the precious little sample at hand, we started with a seemingly simple experiment: watching the protein droplets evaporate! The idea behind the experiment was to make use of the coffee-ring effect, where the solute particles (in our case the proteins along with salts) would get concentrated at the evaporating boundary and thus likely exhibit phase separation. The idea worked remarkably well, giving us beautiful videos of GRP undergoing phase separation to form condensate droplets (Figure 1b). This assay acted as a simple but effective platform for us to build a detailed phase diagram, aided by some microfluidic experiments (our lab’s expertise) from Chang (another PhD student) to show the kosmotropic salt-induced nature of the phase separation (Figure 1c). Encouraged by these findings, we further set to dissect the driving forces behind the observed phase separation. A timely involvement of EnzyTag, a company with expertise in peptide synthesis, came in handy to design GRP fragments and mutants. Manali, a newly arrived postdoc in my lab, also fit in perfectly, and using her expertise in physical chemistry, we systematically identified the main cause of phase separation to be cation-pi and pi-pi interactions, primarily mediated by arginine and aromatic (phenylalanine and tyrosine) amino acid residues (Figure 1D). Given its association with the cement cone, we were thrilled to observe that the GRP condensates exhibited ageing and became more solid-like over time, which was confirmed via FRAP studies (Figure 1E). Not only that, but Manali further performed AFM-based force spectroscopy studies to show that the condensates were sticky in nature and showed four orders higher work of adhesion compared to the negative control (Figure 1F).
To get a shot at the actual system, Ketan teamed up with Emily, a postdoc from the Laboratory of Entomology at Wageningen University. Together they collected ticks from a nearby forest area and dissected them to extract their salivary glands. We were delighted to see the presence of protein-rich droplets in the saliva, hinting that what we were observing in a highly simplified system might also hold true in the natural system.
The beauty of collaboration
Needless to say, this project would not have been possible without patient and sincere efforts of so many people from multiple labs. But I am not just talking about the collaboration between labs, I am also talking about collaborations between Master’s students, PhDs, postdocs, technicians, and group leaders, spanning the entire spectrum, each one doing their own task and complementing each other, ending up with a comprehensive work none of us would have been able to perform on their own.
With our publications, we distil the entire scientific process into a clean, logical story, jotting down the scientific contributions, all of which is of course important. But at the same time, one should not forget the blood and the sweat poured into these stories, the frustrations of failed experiments and the thrill of first results, the luck that is needed and built upon, the team work with a tiring number of drafts and hundreds of emails; to ultimately obtain one simple thing: a story we can be proud of! For me personally, this is one such story, which I will cherish regardless of its academic success. These three years have taught me so much, scientifically but also in terms of project and people management, and I consider this one as a key step in my career. There is one other thing that I re-realized during this time, and that is, science is personal: these are not just proteins but your proteins; it’s not just an analysis but her favourite analysis; it’s not just an experiment, it is his crazy experiment; it’s not just an image, but it’s our amazing, beautiful image!
What next?
For us, this study has opened an entirely new research direction. This publication just scratches the surface of understanding tick adhesion. We have identified numerous other tick GRPs that might be prone to phase separation and show similar amino acid traits as the one we have studied. Apart from finding their role in tick cement cone formation, their biocompatibility and material properties offer exciting opportunities in biotechnology and medicine. For example, could these proteins be engineered further to form fibres, to be used as medical sutures? And could we design anti-tick attachment strategies once we know how the cement cone forms? These are lucrative possibilities, but the fundamental appeal of the research remains equally appealing. Along with other well-known bioadhesives – from spiders, sandcastle worms, and mussels, ticks could be yet another organism whose mystery is about to be unravelled!
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.
Your space to connect: The Polarised light Hub
A new Communities’ space to connect, collaborate, and explore research on Light-Matter Interaction, Optics and Photonics, Quantum Imaging and Sensing, Microscopy, and Spectroscopy!
Continue reading announcement




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in