How to Mend a Broken Cell: Let Experimental Evolution Show You How
Published in Ecology & Evolution

The paper in Nature Ecology & Evolution is here: go.nature.com/2yoiQrR
Quite some years ago, Jeff Chen, a former graduate student of mine at the Department of Molecular Genetics, Ohio State University, made a surprising, if not entirely bizarre, finding that a so-called “ESSENTIAL” (indispensable) splicing factor, Prp28, can be eliminated from the cell and yet the cell remains alive (Chen et al. [2001] Mol. Cell 7, 227–232).
It turns out that such a Prp28-less cell survives because of the presence of another mutation (genetic modifier) in the genome. This Prp28-less cell, although alive, grows very poorly at low temperature, to which we understood the molecular underpinning. Contemplating about this odd finding at the time, we speculated that this might be a “resilience” strategy for cell to cope with imminent crisis, e.g., the sudden inactivation of an essential gene, so that the cell stands a chance to survive and perhaps to even venture into new trajectory for restoring vitality when opportunity arises. Unfortunately, there was no easy way at that time to test this hypothesis.

Years later, entered Shang-Lin Chang (a.k.a. Sean), a postdoctoral fellow in my lab, now at the Genomics Research Center, Academia Sinica, Taipei, Taiwan. Sean, who formerly trained in the field of molecular evolution for his Ph.D. at TIGP (Taiwan International Graduate Program), took up the challenge to test the provocative hypothesis. Sean employed experimental evolution to evolve the Prp28-less cell to see if the barely surviving cell will eventually turn into a wholesomely healthy one. So Sean did a “delete-and-evolve” experiment, in which the Prp28-less cell was evolved over 300 generations under constant selection pressure, i.e., low temperature. The idea is that during these 300 rounds of cell doubling, rare mistakes (mutations) in DNA replication will accumulate and, hopefully, some of which will improve the odds of Prp28-less cell to grow ever better under pressure. Such arguably is a Darwinian evolution process under the laboratory condition.
The outcome of that experiment could not be more astounding. Yes, the Prp28-less cell eventually arrived at a state rivaling that of the wild-type cell. That is, rising up from the Prp28-less “ashes” now stands a new life that no longer needs the once indispensable Prp28. Detailed analysis revealed that the Prp28-less cell accomplishes this feat by tweaking SAGA transcription co-activator complex’s transcription-linked activity. Sean went on to show that purposely slowing down Pol II transcription rescues the splicing defect and improves ancestor’s cellular fitness. In the nutshell, re-synchronization between transcription and splicing contributes to the robustness of the gene-expression pathway.
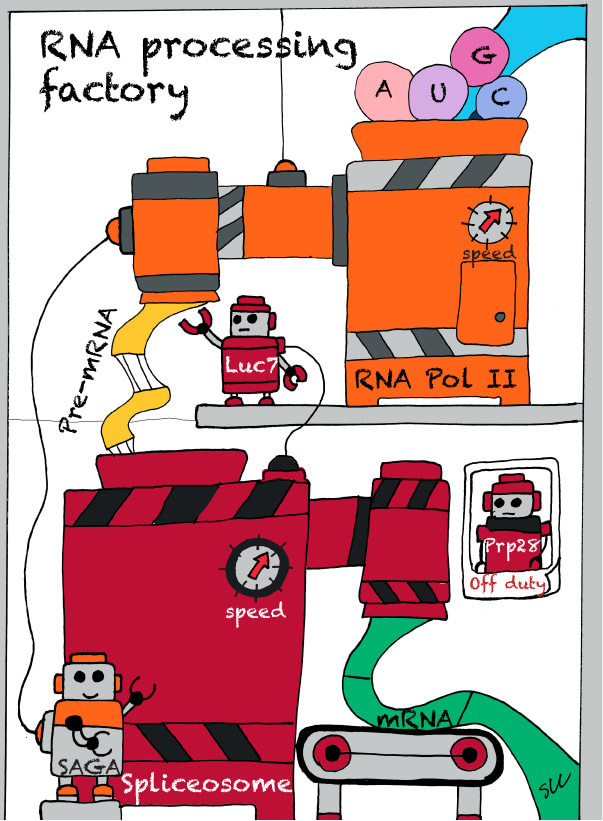
Thus, it looks like cell, in the face of a clear and present "danger", is capable of taking an expedition via evolution to compensate for the loss of an essential component. In the end, a novel genome and system reaches a new fitness peak. Sean’s findings also provide a powerful support that the the transcription and splicing pathways are intimately coupled with each other in vivo, so altering one (transcription) helps to rescue the defects of the other one (splicing).
In a bigger picture, Sean’s study puts forward an interesting idea that the intrinsic interconnectivity within a biological system, which is presumably evolved for the purpose of system robustness, can be exploited for adaptive evolution and system reoptimization. Sean’s story evidently attests to what Theodosius Dobzhansky (1900-1975) once said “Nothing in biology makes sense except in the light of evolution”, in that evolution offers endless possibilities to lay down the foundation of life.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in