Integrated multimodality microscope for accurate and efficient target-guided cryo-lamellae preparation
Published in Protocols & Methods

Resolving the structure of subcellular organelles and macromolecular complexes in the native cellular context is the ultimate goal to understand how life works. Cryo-electron tomography (cryo-ET) is currently a promising technique to achieve this goal. One of the major limitations of cryo-ET is its restricted imaging depth, which is typically a few hundred nanometers. Therefore, when applying cryo-ET to vitrified cells, thinning them to lamellae of not more than 200 nm thickness is necessary. Nowadays, focused ion beam (FIB) milling has become increasingly popular for trimming vitrified biological specimens into thin lamellae. However, conventional FIB milling does not allow site-specific fabrication due to the inability to locate the target of interest underneath the cell. This drawback can be resolved by combining FIB with fluorescence microscopy and performing correlative light and electron microscopy (CLEM). In CLEM, fluorescence imaging is used to identify and locate the target, and to guide FIB milling at specific positions of interest after image registration.
In our publication, we describe a cryogenic correlative light, ion and electron microscopy (CLIEM) system that incorporates a confocal microscope into a commercial FIB-scanning electron microscope (SEM). Using the multimodality CLIEM, it becomes possible to prepare lamellae of vitrified cells under the guidance of fluorescence at specific sites with high accuracy and high efficiency. One of the unique characteristics of our CLIEM system is its high-performance custom-built confocal microscope. First, we reduced the working distance of light microscopy (LM) to 1 mm through the careful construction of the sample holder. This allowed us to use objectives with high numerical apertures up to 0.9 in order to acquire high quality images of whole cells in 3 dimensions (3D). Furthermore, we optimized the light path to achieve a maximum field-of-view (FOV) of 145 μm × 145 μm, and this FOV could be conveniently magnified by altering the scanning angle of the confocal beam without the necessity of changing the objective. Last, we incorporated three simultaneous acquisition channels for multicolor imaging and established bright field imaging in addition to fluorescence imaging.
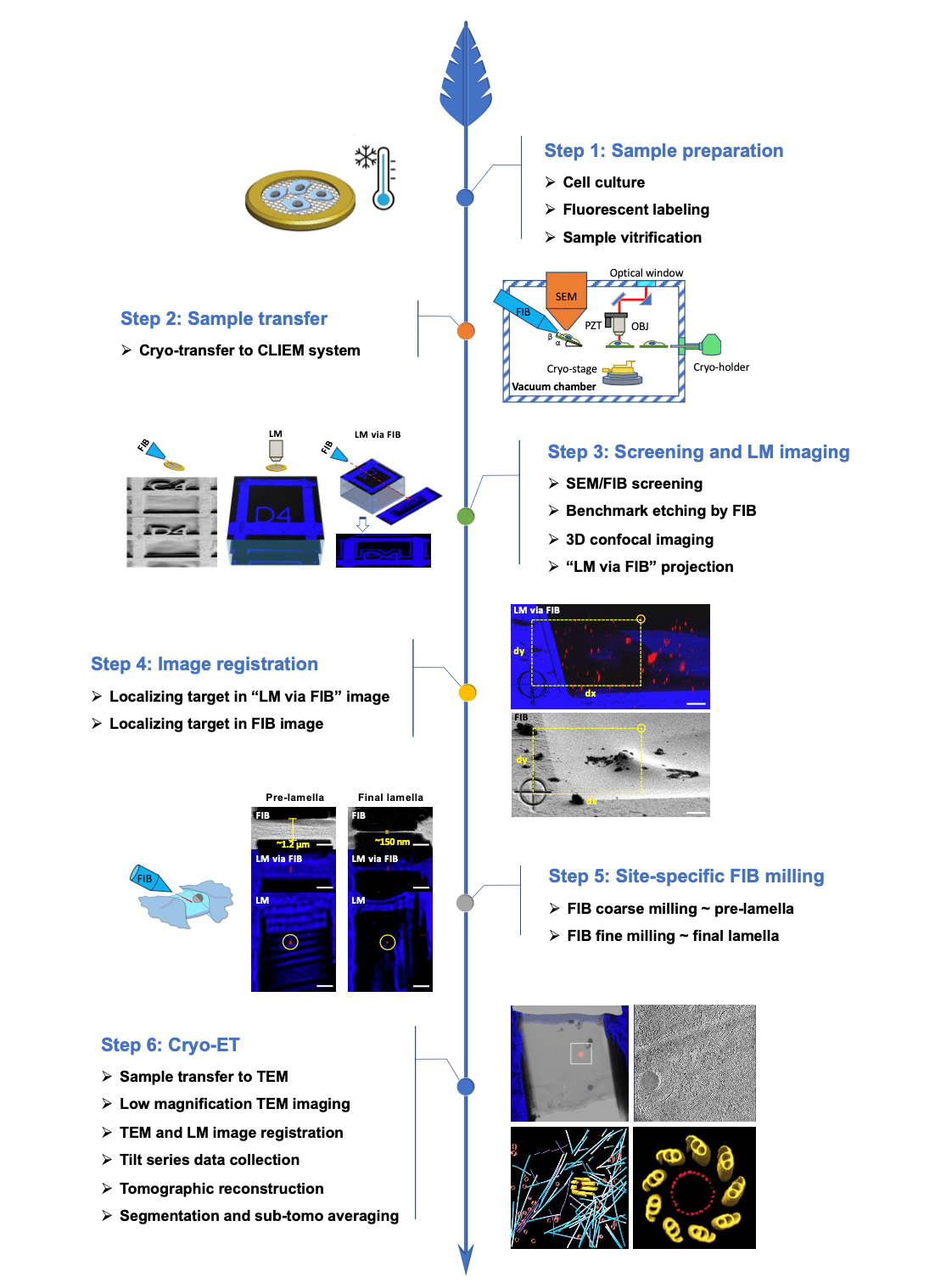
Besides developing new hardware, we also established a dedicated workflow for fluorescence-guided FIB milling. The key steps are shown in Figure 1. We utilized FIB to etch a pattern as the benchmark for image registration. In this way, we were able to circumvent the need for fiducial markers and simplify the sample preparation procedure. Moreover, we introduced a new principle for registering the LM and the FIB images. The 3D confocal image was projected along the milling angle of FIB, and on the projected image, referred to as “LM via FIB”, the distances between the etched benchmark and the target were measured. These distances could be directly used to locate the target on the FIB image. Compared to the traditional image registration process utilizing fiducial markers and coordinate transformation, our workflow features high working efficiency of approximately one hour per lamella, high milling accuracy of less than 50 nanometers, a high success rate of more than 95%, and great ease of use.
Figure 1 Workflow of cryo-ET sample preparation using the cryo-CLIEM system. Step 1: cells are cultured on EM grids, labeled with fluorescent probes, and vitrified by plunge freezing. Step 2: the vitrified samples are transferred to the CLIEM system using a custom-built cryo-transfer system. Step 3: the cells are screened by SEM and FIB imaging, and a benchmark is etched on the grid bar beside the candidate cell. Then the sample is moved to LM position and 3D confocal image is acquired. By projecting the 3D image along the direction of FIB milling, a 2D image referred to as “LM via FIB” is created. Step 4: “LM via FIB” and FIB images are registered, and the target is localized in both images with respect to the etched benchmark. Step 5: a pre-lamella is fabricated by FIB coarse milling at the determined position of the target. Under the guidance of “LM via FIB” of the pre-lamella, the final lamella is prepared by FIB fine milling. Step 6: the sample is transferred to TEM, and a low magnification image is acquired and registered with the LM image to locate the target. Finally, cryo-ET data is collected, reconstructed and analyzed.
We applied the CLIEM to various biological systems. First, we investigated the organelle interactions between lipid droplet and mitochondria, as well as between endoplasmic reticulum and mitochondria. Using our workflow, we successfully prepared cell lamellae containing the contact sites of these organelles and resolved these structures with cryo-ET. Second, using the same protocol, we resolved the in situ structure of centrosome, which is a very rare event in cells. We analyzed the native microtubules, intermediate filaments and transport vesicles around the centriole and confirmed that all the microtubules are oriented out from the centriole. Moreover, we discovered a new ring structure that consists of 27 evenly distributed nodes inside the centriole.
The CLIEM system and dedicated workflow provide new possibilities for targeted fabrication of cell lamella using FIB. With its high optical performance and high milling accuracy, the CLIEM can be applied to a wide variety of biological systems, ranging from multicolor labeled organelle interactions to rare cellular events such as centrosome. As an additional benefit, our integrated confocal microscope was designed as an independent add-on module, and most commercial FIB-SEM systems can be modified to use our confocal microscope and the CLIEM workflow. With our CLIEM method, we hope to offer an all-in-one solution for preparing cryo-lamellae of vitrified cells at specific sites and promote more applications in the field of in situ structural biology with cryo-ET.
Follow the Topic
-
Nature Methods

This journal is a forum for the publication of novel methods and significant improvements to tried-and-tested basic research techniques in the life sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Methods development in Cryo-ET and in situ structural determination
Publishing Model: Hybrid
Deadline: Jul 28, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in