Longitudinal liquid biopsy predicts clinical benefit from immunotherapy in advanced non-small cell lung cancer
Published in Cancer, Biomedical Research, and General & Internal Medicine
Our recent study published in npj Precision Oncology examined the use of longitudinal liquid biopsies to predict clinical outcomes in patients with advanced non-small cell lung cancer (NSCLC) undergoing immunotherapy.
We conducted a prospective study involving 113 advanced NSCLC patients treated with immune checkpoint inhibitors (ICIs). We performed liquid biopsies at three time points:
- T1: At the start of ICI treatment
- T2: After three/four weeks at the end of first cycle of treatment
- T3: At the time of radiological evaluation
The study focused on several molecular variables:
- cfDNA quantification: Measuring the amount of circulating free DNA
- ΔT2-T1 cfDNA: The change in cfDNA levels between T1 and T2
- maxVAF: The variant allele frequency of the gene with the highest frequency detected at baseline using next-generation sequencing
- ΔT2-T1 maxVAF: The change in maxVAF between T1 and T2
Key findings include:
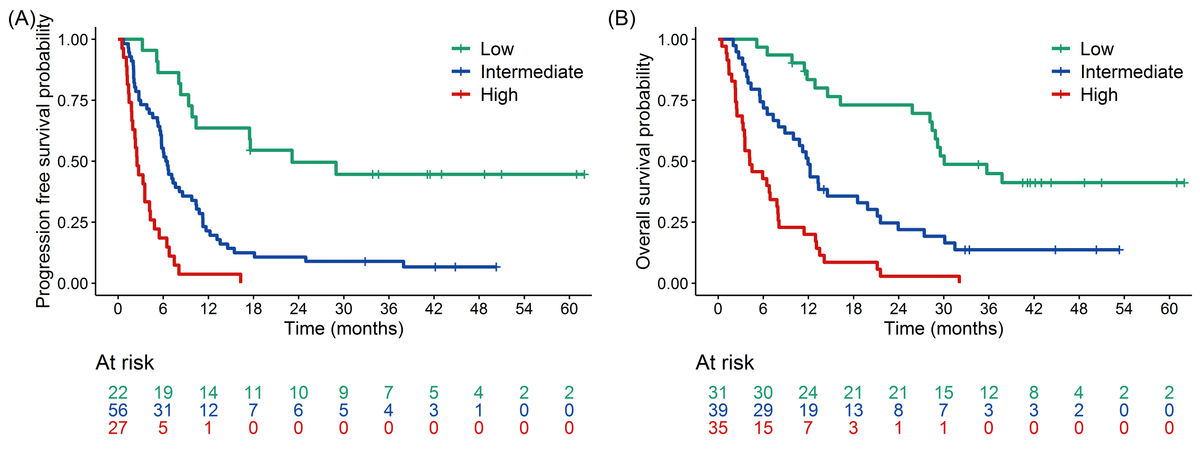
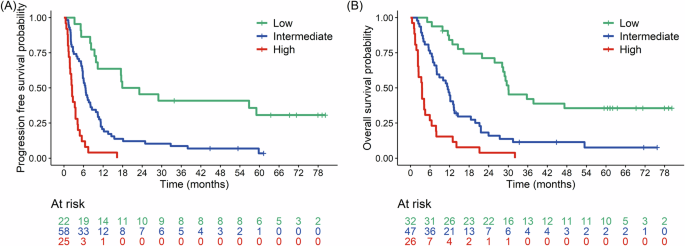
- Progression-Free Survival (PFS): Shorter PFS was significantly associated with PD-L1 negativity, higher cfDNA levels at T1, an increase in cfDNA between T1 and T2, and higher maxVAF at T2.
- Overall Survival (OS): Factors linked to shorter OS included PD-L1 negativity, squamous histology, elevated cfDNA at T1, an increase in cfDNA between T1 and T2, and higher maxVAF at T2.
The study developed a composite prognostic model combining molecular biomarkers (e.g., cfDNA levels, changes in maxVAF) with clinical factors such as PD-L1 expression and tumor histology. This integrated model demonstrated superior predictive accuracy for progression-free survival (PFS) and overall survival (OS) compared to molecular or clinical metrics alone.
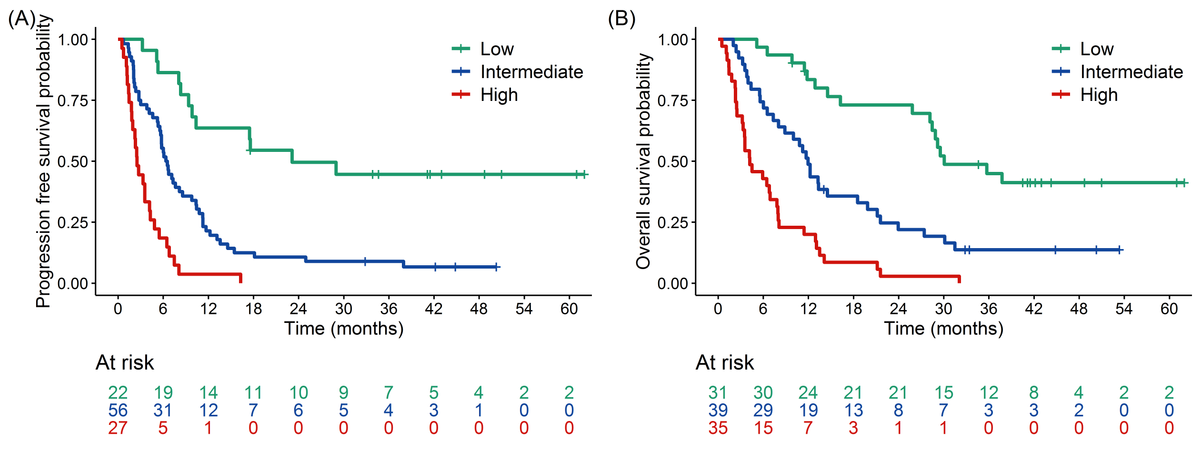
The integrated approach enables better risk stratification and personalized treatment planning for NSCLC patients undergoing immune checkpoint inhibitor therapy.
This study highlights the potential of combining liquid biopsy metrics with clinical data to refine prognostic tools for immunotherapy response.
Follow the Topic
-
npj Precision Oncology

An international, peer-reviewed journal committed to publishing cutting-edge scientific research in all aspects of precision oncology from basic science to translational applications to clinical medicine.
Related Collections
With Collections, you can get published faster and increase your visibility.
AI Approaches in Drug Design
Publishing Model: Open Access
Deadline: Mar 31, 2026
Genomic Instability
Publishing Model: Open Access
Deadline: Mar 24, 2026
Latest Content
Why is Singapore Identified in Global Research as Number One? How Physical Activity and Education Excellence Created a Global Leader




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in