Integration of Pathology Subspecialties: From Bedside to Bench and Back
Published in General & Internal Medicine
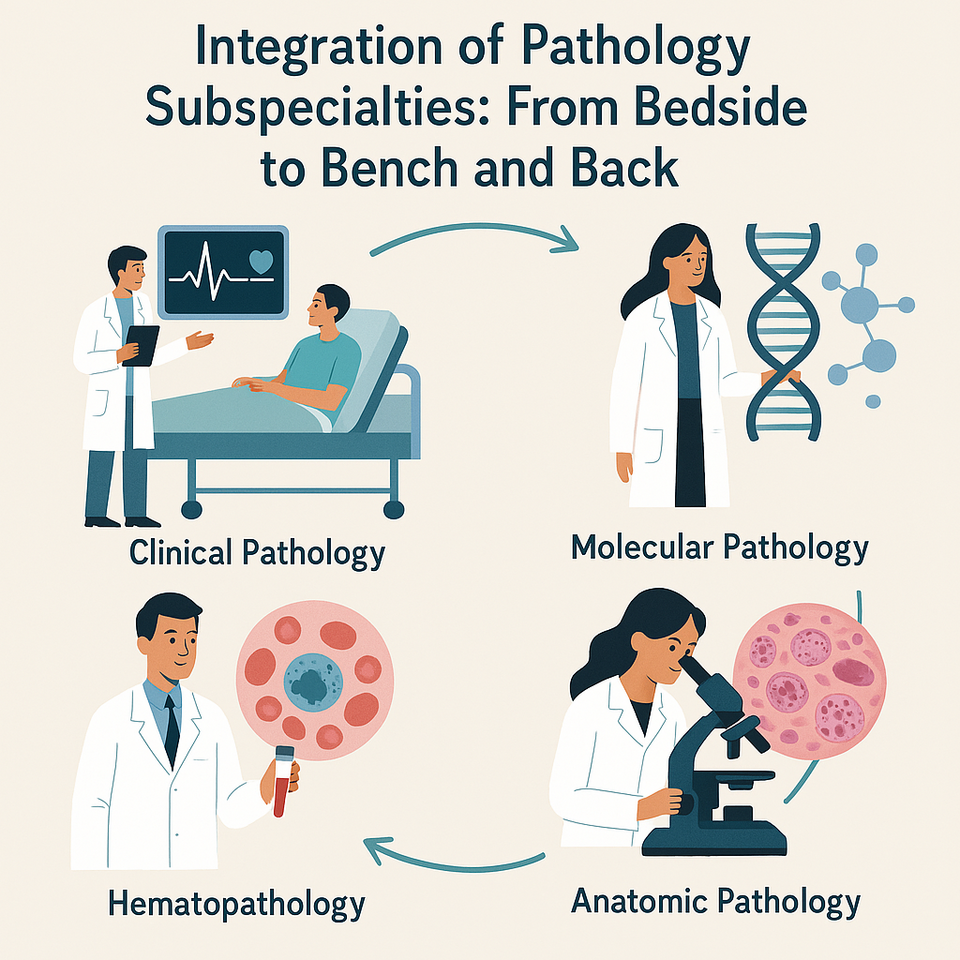
Pathology, often referred to as the “science of disease,” is not a singular field but a constellation of highly specialized domains, each contributing uniquely to the diagnosis, prognosis, and management of diseases. The evolving complexity of medicine, emergence of molecular diagnostics, and precision therapies have necessitated a more integrated approach one that moves seamlessly from the patient’s bedside to the research bench and back again. This is the essence of translational pathology.
How Many Specializations Are There in Pathology?
Pathology is broadly classified into two main domains:
-
Anatomical Pathology (AP):
-
Histopathology: Microscopic examination of tissues.
-
Cytopathology: Study of cells (e.g., Pap smears, FNAC).
-
Forensic Pathology: Investigating cause of death, post-mortem analysis.
-
Neuropathology: Specialization in diseases of the nervous system.
-
Dermatopathology: Skin diseases at the microscopic level.
-
Head and Neck/Oral Pathology: Diagnosis of tumors, infections, and premalignant lesions in the oral and maxillofacial region.
-
-
Clinical Pathology (CP):
-
Hematopathology: Blood, bone marrow, and lymphatic disorders.
-
Clinical Chemistry: Blood glucose, enzymes, electrolytes, etc.
-
Microbiology: Bacteriology, virology, parasitology, mycology.
-
Immunopathology: Autoimmune disorders, immune-mediated diseases.
-
Molecular Pathology: DNA/RNA-based diagnostics (PCR, NGS, etc.)
-
Genetic/Genomic Pathology: Inherited disorders, prenatal testing.
-
Transfusion Medicine: Blood banking, immunohematology.
-
Chemical Pathology: Toxicology, metabolic disorders.
-
Infectious Disease Pathology: Pathogen detection and resistance profiling.
-
The integration of various pathology subspecialties is essential to constructing a holistic and accurate diagnostic profile. In contemporary clinical practice, these departments do not function in isolation; rather, they synergistically contribute to diagnostic clarity through a systems-based approach. For instance, in cancer diagnostics, a tissue biopsy is first examined through histopathology using H&E staining, followed by immunohistochemistry (IHC) to determine tumor origin, and then further analyzed via molecular pathology to identify actionable genetic mutations such as EGFR or KRAS. Similarly, infectious disease workups exemplify the collaboration of microbiology, molecular pathology, and hematology where microbial cultures may identify causative organisms, PCR detects pathogen-specific genetic material, and hematological assessments evaluate the systemic impact. In hematologic malignancies such as chronic myeloid leukemia (CML) or acute lymphoblastic leukemia (ALL), cytopathology, immunophenotyping via flow cytometry, and genetic testing through FISH or gene panels work in tandem to reach a precise diagnosis and stratify prognosis.
This multidisciplinary orchestration extends into translational medicine, enabling a seamless flow from bedside to bench and back. When a patient presents clinically say, with persistent oral ulceration or unexplained lymphadenopathy the clinician initiates investigative pathways through appropriate sample collection: biopsies are routed to histopathology, fluids to cytology, and blood to hematology and molecular labs. In the diagnostic phase, each sample undergoes comprehensive evaluation: anatomical pathology assesses tissue morphology, IHC or flow cytometry defines cellular phenotypes, molecular techniques such as qPCR or NGS detect genetic abnormalities, and clinical chemistry or microbiology contributes functional and infectious insights. These findings are collectively interpreted, often through multidisciplinary tumor boards or molecular review panels, to finalize a patient-specific diagnosis and therapeutic plan.
When unusual mutations or drug resistance patterns are encountered, this integrated workflow naturally extends into research domains. Such discoveries may drive retrospective analyses, biomarker validation, or the development of patient-derived translational models like organoids or xenografts. These efforts not only fuel basic science but also contribute to clinical trials and innovations in targeted therapy. Ultimately, this research is brought back to the bedside, where novel biomarkers such as PD-L1 or microsatellite instability (MSI-H) guide the selection of immune checkpoint inhibitors like pembrolizumab. In this way, bench-to-bedside application reinforces the loop of precision medicine.
The clinical implications of this integrative model are profound. It enables faster diagnostic turnaround, guides rational test utilization, supports evidence-based targeted therapies, and improves patient outcomes across a wide spectrum of diseases ranging from cancer and infections to autoimmune and inherited disorders. Thus, pathology is no longer a passive, descriptive science confined to slides and stains. It is the backbone of personalized medicine, playing a pivotal role in clinical decision-making, therapeutic stratification, and public health interventions. From tissue architecture to genomics, from microscopic morphology to immunogenomics, pathology acts as the unifying compass of modern medicine navigating uncertainty with scientific precision and clinical relevance.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in