Isolable dicarbon stabilized by a single phosphine ligand
Published in Chemistry

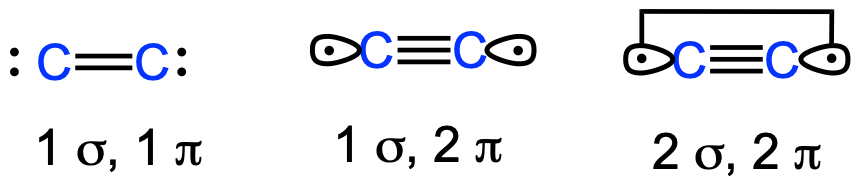
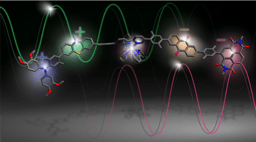
Dicarbon(0) C2, the smallest diatomic molecule with a carbon–carbon bond. In contrast to the natural occurrence of F2, O2 and N2 as diatomic species, C2 is too reactive for experimental studies in the condensed phase, which has been only detected in the blue flame of candle lights and in interstellar atmosphere. The nature and strength of the bond in C2 has proven controversial and there have been numerous experimental attempts to stabilize C2 experimentally with a molecular motif of L→C2←L or L→C2→M ( L = organic donor ligands, M = metal complex). However, the bond length and the bonding situation of the central C2 differ significantly from those of free C2.
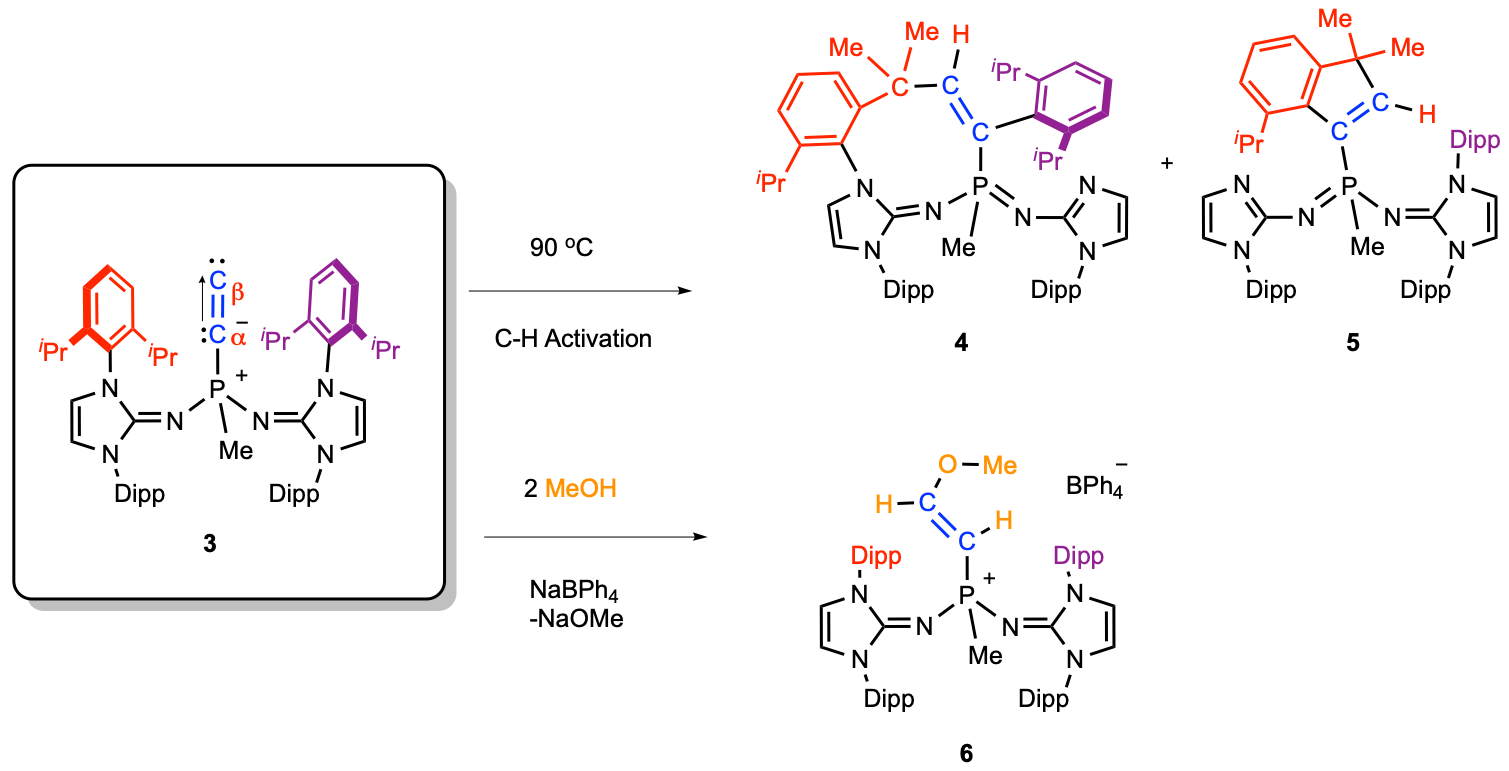
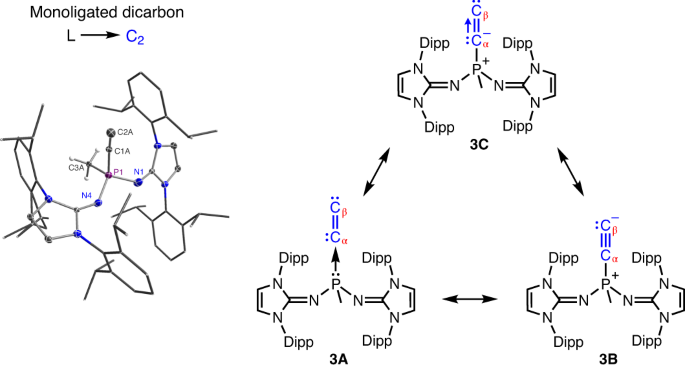
Recently, collaborative research efforts led by Prof. Tiow-Gan Ong at Academia Sinica-Taipei worked hand-in-hand with theoreticians Prof. Gernot Frenking at Marburg University-Germany and Prof. Lili Zhao at Nanjing Tech University-China to isolate and understand a more stable and naked C2 molecule as L→C2 using bulky phosphine ligand bearing two imidazolidin-2-iminato groups (L is (NHCR=N)2(CH3)P, where NHCR is a N-heterocyclic carbene). Three possible formal resonance forms as 3A, 3B and 3C have been suggested for R3P→C2 (Figure 2). In the crystal structure analysis of R3P→C2, the observed Cα-Cβ bond distance in the carbenoid ligand, with 1.237(4) Å, is only marginally shorter than in free C2 (1.2425 Å) and intermediate between typical Csp1-Csp1 triple bonds 1.183(14) Å and unconjugated Csp2-Csp2 double bonds, 1.478 (12) Å. The computational analysis based EDA-NOCV (Energy decomposition analysis combined with natural orbitals for chemical valence) was also employed, indicating P–Cα bond has fragments of C2- and the phosphine+ ligand lied toward behaving more like resonance structure of C than A and B.

The chemical reactivity of R3P→C2 also showed that the formation of the L→C2 bond induces a large charge migration towards C2, and that the two carbon atoms of the C2 moiety show carbene character, leading to intermolecular C–H bond activation upon thermolysis and the reaction with methanol via formal O–H bond insertion. We hope that this isolation of a stable complex of C2 will offer possibilities for further new chemical bonding concept in main-group and transition-metal chemistry with applications.
Learn more about our discovery published at Nature Chemistry (2020). https://www.nature.com/articles/s41557-020-00579-w
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in