Ketogenic diet in colorectal cancer - just how powerful is the gut microbiome?
Published in Cancer and Microbiology
Colorectal cancer is the second most common form of cancer worldwide1. Concerning as this already sounds, the breakdown of these statistics reveals a far more worrying trend – while the CRC-related death rate has been steadily dropping in the older population (over 55 years of age), thanks to advances in screening, the death rate amongst the younger population has risen by about 1% per year for the past 20 years2. Among the various lifestyle-related risk factors associated with CRC – dietary habits2. But how can the food we consume affect CRC development? While direct effects are also likely at play, the far more elusive indirect effects of diet on CRC piqued our interest. Our main suspect – changes in the gut microbiome as a mediator of inflammation3–6.
The gut microbiome is the ensemble of micro-organisms living in the gastro-intestinal tract and is a key player in shaping immune responses and overall well-being. Incidentally, some bacteria living in us have been shown to possess cancer-driving properties, while others have been linked to gut health7,8. Diet is an important modulator of the gut microbiome and its effects can be dependent on the composition and function of the microbiome9,10. In our recent manuscript, we demonstrate that the gut microbiome plays an important role in mediating the effects of ketogenic diet – a high-fat low-carbohydrate diet, long investigated for its beneficial effects in various cancer types, but whose effects were still poorly described in CRC11.
The ketogenic diet had been described both as beneficial and as detrimental in different cancer contexts, including CRC, as well as in the context of other diseases11,12. So far with no clear underlying cause for the discrepancy – which for us constituted first hint towards a potential role of the microbiome in mediating its beneficial effects. That is why we - in the midst of a hype on dietary fiber modulation - set our sights on the ketogenic diet. And what started out as a descriptive study of gut microbial changes over time, rapidly evolved into the pursuit of gut microbiome modification through dietary intervention in an attempt to modulate disease outcomes.
But how does one address all of this? As when one is trying to establish a standard operating protocol through trial and error, the “recipe for success” only becomes clear in hindsight, once many avenues have been followed and ruled out, and a mound of negative data has accumulated along the way.
Of utmost importance, the selection, establishment and characterization of an appropriate model of study. For this study, we established an inflammation-driven mouse model of CRC transplanted with a human gut microbiome to create a model more relevant to human disease. And this, in our brand new (at the time) germ-free animal facility at the University of Luxembourg – only possible with the constant support of all staff members and the members of our research group!
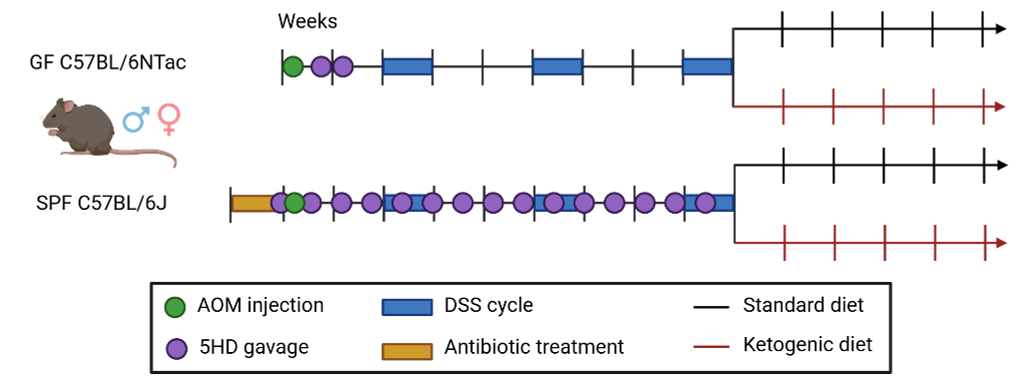
Figure 1. | Experimental design. Humanization of the murine gastro-intestinal tract, followed by the induction of colorectal cancer. Created with Biorender, from Tsenkova et al. 2025.
We were able to observe changes in gut microbial composition over time in response to the ketogenic diet, as well as a reduced colonic tumor burden at endpoint, demonstrating the beneficial effects of the diet in this model, and the accompanying response of the gut microbiome. But were these changes in any way causal, or were they a mere “side effect” of the consumption of ketogenic diet?
And here is the next key moment – designing an experiment to test this hypothesis. Now much more adept in microbiome transplantation, we transplanted the microbiomes of ketogenic-diet-fed mice and of standard-diet-fed mice into a set of recipient germ-free mice undergoing the same cancer model and observed similar results. The gut microbiome alone, previously shaped by ketogenic diet, and without continued consumption of ketogenic diet, was sufficient to reduce colonic tumor burden in the recipient mice. Interestingly, the effect was not as pronounced as in the original ketogenic-diet-fed donor mice, with a varying strength of response, leading us to further hypothesize that variations in the gut microbiome may define responders and non-responders to dietary intervention, potentially accounting for the dissent in experimentation involving the benefits ketogenic diet in cancer – to be continued!
And finally, the most important thing of all – recruiting the right people at the right time to push the mission forward. While some members were fully invested in the project from its infancy, others joined the group along the way, forming a formidable team. Our team’s efforts really drove the project home in the final year, honing in on a single metabolite which was highly upregulated in the gut of mice consuming the ketogenic diet. This metabolite – stearate, a free long chain fatty acid - showed both direct effects on CRC cells, by inducing cell death, and indirect effects, by reducing the abundance of a subset of detrimental immune cells in the gut and most importantly by inhibiting tumor growth in our humanized microbiome mouse model.
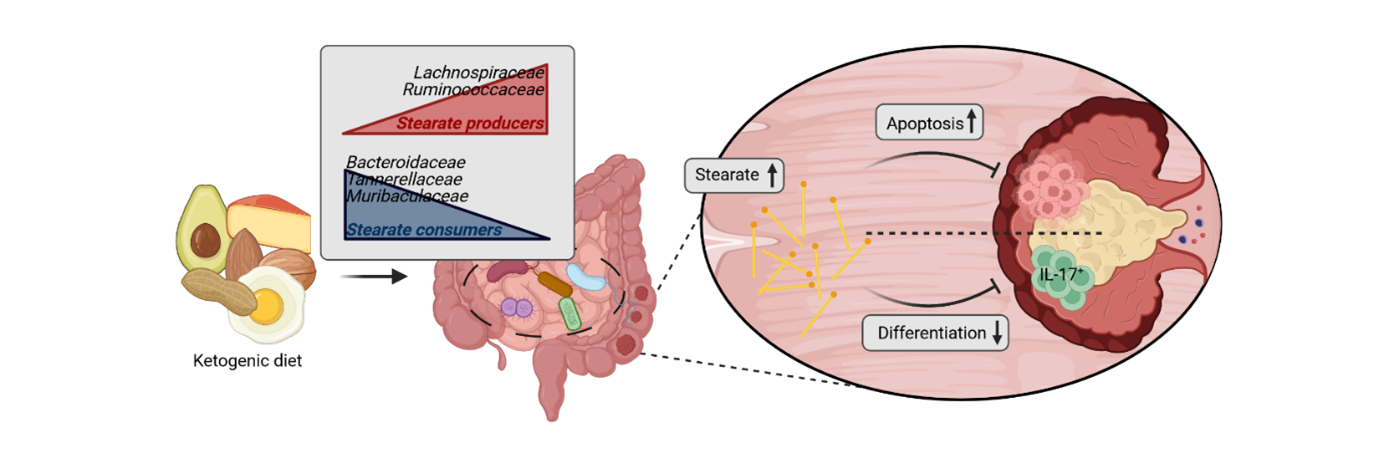
Figure 2. | Project summary. Ketogenic diet suppresses CRC through modulation of the gut microbiome, favoring an increase in available stearate in the gut lumen, which in turn induces cancer cell death and altered immune responses. Created with Biorender, from Tsenkova et al. 2025.
And so, nearly five years after we set out on this journey, it has come to an end, with the publication of our research findings. We demonstrated the causal role of the gut microbiome in ensuring the beneficial effects of the ketogenic diet, and highlighted the role of one of the metabolites mediating these effects. But is this really the end of the journey? With recent advances in both technology and knowledge, we believe that the time to harness the power of diet and the gut microbiome in improving disease and accompanying traditional forms of treatment is upon us.
Co-authored by: Mina Tsenkova and Madita Brauer
References
- Colorectal cancer statistics. WCRF International https://www.wcrf.org/cancer-trends/colorectal-cancer-statistics/ (2022).
- Akimoto, N. et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol 18, 230–243 (2021).
- Ang, Q. Y. et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 181, 1263-1275.e16 (2020).
- Kong, C. et al. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. Signal Transduct Target Ther 6, 154 (2021).
- Routy, B. et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
- Gopalakrishnan, V. et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
- Wong, C. C. & Yu, J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol 20, 429–452 (2023).
- El Tekle, G. & Garrett, W. S. Bacteria in cancer initiation, promotion and progression. Nat Rev Cancer 23, 600–618 (2023).
- Zhang, Y. et al. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res 145, 163–168 (2018).
- Ross, F. C. et al. The interplay between diet and the gut microbiome: implications for health and disease. Nat Rev Microbiol 22, 671–686 (2024).
- Weber, D. D. et al. Ketogenic diet in the treatment of cancer – Where do we stand? Molecular Metabolism 33, 102–121 (2020).
- Zhu, H. et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Sig Transduct Target Ther 7, 11 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in