Lipid Switches Govern GABAAR Activation—and Alzheimer’s Hyperexcitability
Published in Biomedical Research

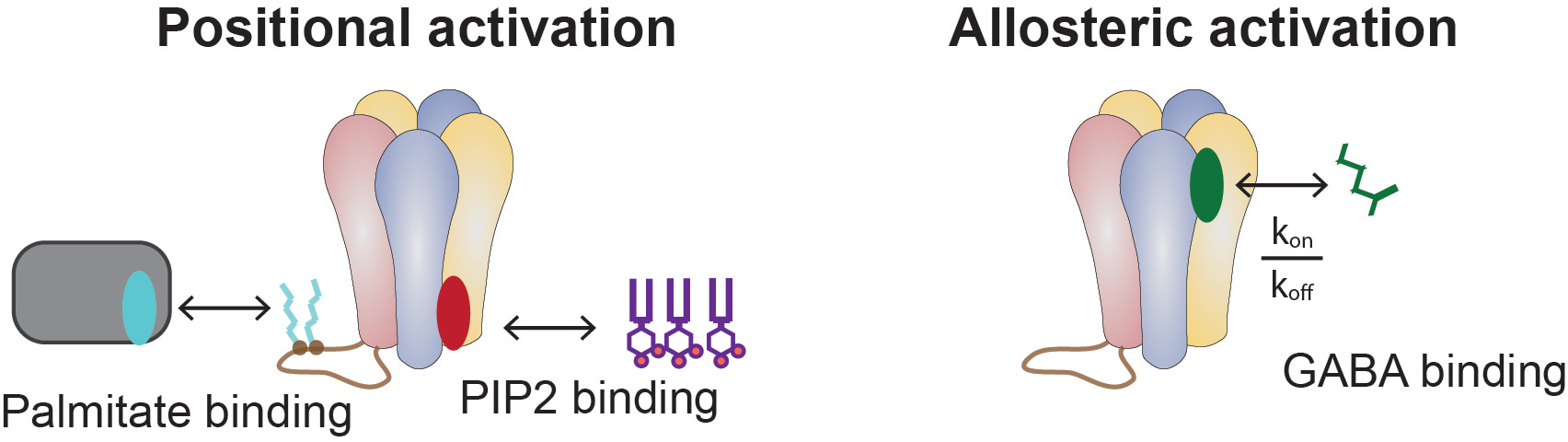
Positional Activation: A New Paradigm
While studying how anesthetics reposition proteins to activate them1, we wondered: Could ion channels like GABAAR obey similar rules? GABAAR was already known to have two key features:
- Palmitoylation (attachment to saturated lipids).
- Binding to PIP2 (an unsaturated lipid).
But their combined role—a toggle between lipid domains dictating receptor activity—was unexplored. Using super-resolution imaging, we saw GABAAR physically shift from saturated to unsaturated lipids upon activation (Figure 1). Crucially, this movement was triggered not just by anesthetics, but by GABA itself, suggesting positional regulation is intrinsic to neurotransmission.
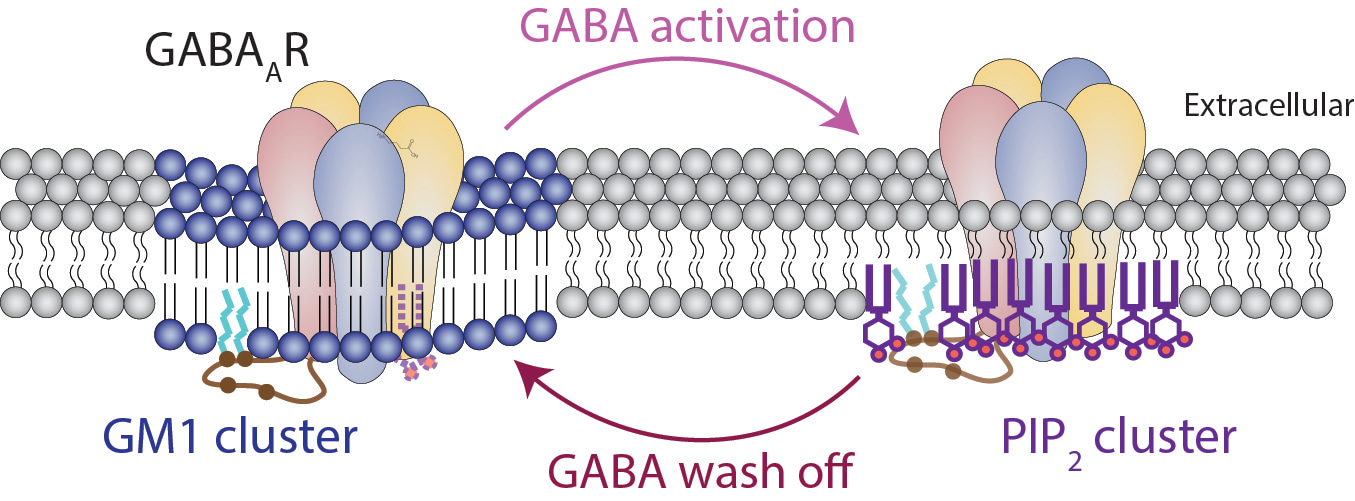
To test if this movement mattered, we used electrophysiology to measure the receptor’s activity. When we added cholesterol from astrocytes—star-shaped brain cells that secrete it via apolipoprotein E (ApoE)—the receptor’s behavior changed. It stayed longer in lipid rafts, delaying activation but boosting the maximum current. This suggested GABAAR is primed for activation by first a visit to lipid rafts, then activation, and ending up bound to PIP2 zones, a novel twist on how neurotransmitters work.
The real-world relevance hit home when we studied an AD mouse model. AD brains have high cholesterol in cell membranes, trapping GABAAR in lipid rafts. This delays the receptor’s calming effect, letting calcium flood neurons and making the brain hyperexcitable—a hallmark of AD. Over time, this ion overload could stress cells, contributing to neuronal death.
One challenge was imaging fast enough to catch this dance in live cells. Our dSTORM technique, while powerful, needed tweaking to balance speed and resolution. Fixed-cell imaging showed lipid clustering, but live imaging confirmed our findings, ruling out artifacts.
Implications for the future.
To understand the importance of this finding consider a sports game like football. Imagine playing a game of football (soccer in the U.S.) and failing to realize that a goal keeper is part of the game. Your team shoots the ball and it almost never goes in the net, and the other team shoots and it almost always goes in the net. Success hinges on understanding the whole picture. Endless training isn’t going to overcome not understanding the concept of a goal keeper.
And so it it with GABAAR. We can give GABA, but sometimes the receptor doesn’t open, for example in a purified membrane. Other times, e.g., in the presence of an anesthetic like propofol it open without GABA. Up until now we have only thought about direct allosteric activation by GABA , we’ve been missing a player, in this case an opportunity to regulate or potentially active the receptor based on its position
This discovery opens new doors. The receptor’s lipid dance might explain how anesthetics calm the brain, mimicking a natural process. It also highlights cholesterol as a signaling molecule, with implications for mood disorders and AD therapies. We’re now exploring how to target this mechanism, hoping to turn our unexpected detour into new treatments.
References
- Pavel, M. A., Petersen, E. N., Wang, H., Lerner, R. A. & Hansen, S. B. Studies on the mechanism of general anesthesia. Proc Natl Acad Sci U S A 117, 13757–13766 (2020).
- Wang, H. et al. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc Natl Acad Sci U S A 118, e2102191118 (2021).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in