Mass photometry-a tool for detecting and quantifying nanoscale protein phase separation
Published in Protocols & Methods

Multivalent, transient interactions under given solution conditions (e.g., pH, ionic strength, temperature, macromolecular crowding) dictates whether a protein will spontaneously phase separate into two phases—a dense phase comprising of spherical protein rich droplets and a dilute phase. For given protein concentrations, the interdependent and combinatorial effects of these parameters define a phase boundary for the protein. When quenched inside this two-phase regime and at moderate supersaturation, the formation of the spherical phase separated assemblies are governed by classical homogeneous nucleation theory1. Here, the kinetics of formation of stable nuclei is rate limiting, but when they form (generally within milliseconds in a conducive environment), their subsequent maturation into larger, macroscopic spherical condensates is a rapid and energetically downhill process. Interestingly, phase separation behavior of the Parkinson’s disease associated protein, alpha-synuclein (a-Syn) does not always seem to follow these principles. One prominent deviation arises from the fact that even in sub-saturated concentrations (close to the two-phase boundary); the protein can form condensate droplets—however, with a substantially delayed kinetics ranging from hours to days depending on solution conditions2-4.
This peculiar behavior of a-Syn phase separation made a curiosity driven team of biophysicists (us, Picture #1) led by Prof. Alexander K. Buell to discern this phenomenon in more depth and detail. After some brainstorming sessions, we finally arrived at two hypotheses which we wanted to experimentally probe in our study: (1) There is a large initial nucleation energy barrier for a-Syn monomers to undergo phase separation. (2) Initial pre-nucleation clusters form spontaneously but their maturation into macroscopic droplets is a slow process. Either way, the apparent saturation concentration at which the protein underwent phase separation had to be re-defined. Initially the presence of stable, prenucleation clusters seemed unlikely and we undertook a ‘proof by contradiction’ approach where we wanted to prove that stable, pre-nucleation clusters did not exist. We planned to work on the first hypothesis and try to modulate conditions that will alter the energy barrier leading to spontaneous demixing—allowing us to understand the thermodynamics of the delayed phase separation conditions. But Alexander’s intuitions said otherwise. He envisioned that traditional microscopic or light scattering approaches might not detect the pre-nucleation clusters if they account for a vanishingly small volume fraction.
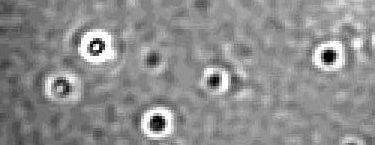
During this time, Alexander visited his old colleague, Nikolai Lorenzen at Novo Nordisk and was introduced to mass photometry (Refeyn)5. The OneMP version of this instrument allows label-free, single molecule mass measurements in the range of ~40kDa-5MDa. This was exactly what we needed! The cut-off was conveniently placed so that monomeric a-Syn (~15kDa) molecules were invisible in the concentration regime in which we wanted to probe existence of pre-nucleation clusters (if any). Meaning that small a-Syn assemblies larger than 40kDa could be detected and quantified without the background signal from the monomeric population. And in November 2021, we had our first ‘eureka’ moment. Sub-saturated concentrations of a-Syn where we could not detect macroscopic phase separation for hours/days, showed spontaneous (within seconds) formation of nanoscale clusters in mass photometry (Figure #1). These nanoclusters had a defined existence boundary in the sub-saturated regime and contained tens to hundreds of molecules depending on total a-Syn concentration. These assemblies responded to external tweaks the same way as a macroscopic droplet would. Importantly, the nanoclusters were thermodynamically less stable than a-Syn oligomers—making a strong case for them being phase separated assemblies, just smaller. And thus, our second hypothesis was proven to be correct, at least partially. The next challenge was to explain why the nanoclusters were stable in time. Why would they not rapidly grow into macroscopic droplets?
I had several rounds of discussions with Thomas, Lars and Alexander about whether these nanoclusters could be stable, micellar structures. We hypothesized that if the nanoclusters resembled micelles, the surface of the nanoclusters could be enriched with the acidic (negatively charged) C-terminal of a-Syn—preventing further monomer incorporation and slowing down growth/fusion by electrostatic repulsion. During this time, Azad was working with mutational screens of a-Syn to delineate the effects of C-terminal charges on its amyloid aggregation. He had generated a unique mutant where five C-terminal acidic residues (negatively charged glutamic acid) were substituted by basic ones (positively charged lysine). Strikingly, a comparative, orthogonal phase separation screen showed that this mutant could phase separate into macroscopic assemblies at concentrations where the wild-type a-Syn could only form nanoclusters. We were intrigued by this result since it was direct evidence in support of our working model. However, the one dataset that puzzled us (and still does) was that the mass of the nanoclusters increased systematically with increasing total a-Syn concentration. In a typical micellar system, one would expect a constant size of micelles above the critical micellar concentration (CMC). We intend to find answers to this in a future study.
In a broader context, I believe a-Syn phase separation is an important area of future research. Until recently, it was believed that nucleation of a-Syn amyloid aggregation was exclusively surface (e.g., lipid membranes) or interface (e.g., air-water or oil-water) dependent6,7. This exclusivity was challenged when a-Syn was reported to undergo phase separation and promised a new pathway of amyloid fibril formation associated with Parkinson’s disease4. Now we show that a-Syn nanoclusters can also efficiently accelerate amyloid fibril formation even at physiological concentrations which might have crucial pathological significance. In addition, we establish mass photometry as a promising tool to detect and quantify nanoclusters in sub-saturated concentrations, which is now emerging as a rather generic phenomenon for proteins that undergo phase separation8. Our reviewers helped us massively to improve this body of work, which is now published in Nature Chemistry (DOI: https://doi.org/10.1038/s41557-023-01244-8).

References:
1 Martin, E. W. et al. A multi-step nucleation process determines the kinetics of prion-like domain phase separation. Nature Communications 12, 4513, doi:10.1038/s41467-021-24727-z (2021).
2 Ubbiali, D. et al. Direct Observation of “Elongated” Conformational States in α-Synuclein upon Liquid-Liquid Phase Separation. Angew. Chem. Intl. Ed. 61, e202205726, doi:10.1002/anie.202205726 (2022).
3 Takamuku, M. et al. Evolution of α-synuclein conformation ensemble toward amyloid fibril via liquid-liquid phase separation (LLPS) as investigated by dynamic nuclear polarization-enhanced solid-state MAS NMR. Neurochemistry international 157, 105345, doi:10.1016/j.neuint.2022.105345 (2022).
4 Ray, S. et al. α-Synuclein aggregation nucleates through liquid–liquid phase separation. Nature Chemistry 12, 705-716, doi:10.1038/s41557-020-0465-9 (2020).
5 Young, G. et al. Quantitative mass imaging of single biological macromolecules. Science 360, 423-427, doi:doi:10.1126/science.aar5839 (2018).
6 Campioni, S. et al. The presence of an air-water interface affects formation and elongation of α-Synuclein fibrils. J Am Chem Soc 136, 2866-2875, doi:10.1021/ja412105t (2014).
7 Galvagnion, C. et al. Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nature Chemical Biology 11, 229-234, doi:10.1038/nchembio.1750 (2015).
8 Kar, M. et al. Phase-separating RNA-binding proteins form heterogeneous distributions of clusters in subsaturated solutions. Proc. Nat. Acad. Sci. 119, e2202222119, doi:doi:10.1073/pnas.2202222119 (2022).
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in