Mastering Cardiomyocyte Production: From Complex Bioreactors to Scalable Spinner Flask Systems
Published in Bioengineering & Biotechnology, Anatomy & Physiology, and Mechanical Engineering
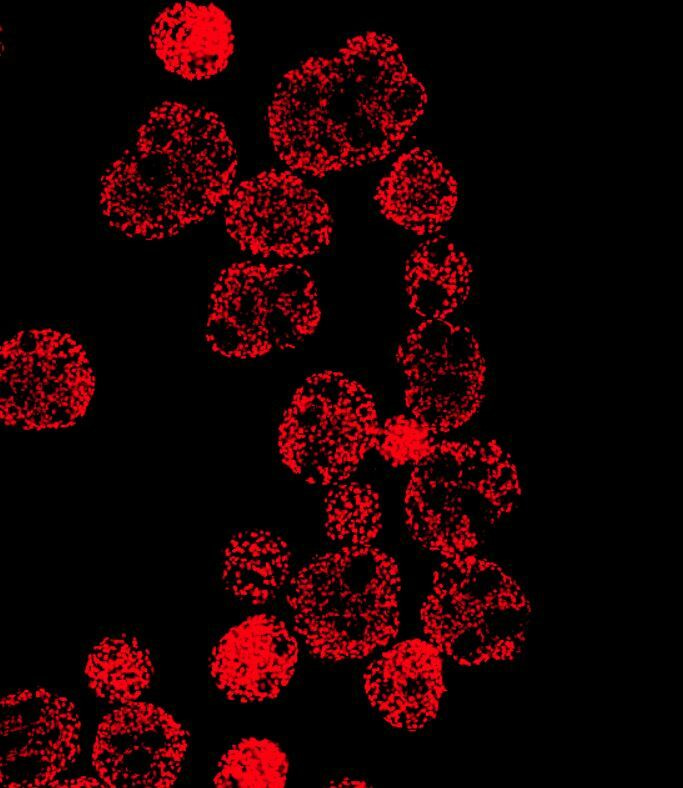
Whenever someone asks me about my work, the production of cardiomyocytes from human pluripotent stem cells (hPSCs) in stirred bioreactor systems, I will quickly start to talk about the finicky, technical details of such systems, which are one of the main reasons for my fascination towards them.
However, we understand that not everyone likes these sometimes-moody devices, as their handling requires a very special skill set and probably a special mindset as well. Often, collaborators approach us asking for simpler ways to produce hPSC-derived cardiomyocytes (hPSC-CMs) in meaningful quantities in suspension culture. For this very reason, we have developed a protocol to produce hPSC-CMs in so-called spinner flasks, which are relatively simple, typically single-use, and affordable stirred bioreactors. Although the control of process parameters such as pH or dissolved oxygen is omitted for simplicity reasons, stirred spinner flask systems still allow the important control of hPSC aggregation via the stirring speed and promote process upscaling in suspension culture.
The production of hPSC-CMs has come a long way in our group, which led to the publication of earlier manuscripts in Nature Protocols. First, the cultivation of hPSCs as matrix-free cell-only aggregates yet – importantly - avoiding the unwanted spontaneous differentiation was demonstrated (Zweigerdt et al., 2011). This was followed by the directed differentiation into hPSC-CM in stirred suspension culture at relatively high lineage purity (Kempf et al., 2015) and more recently into a scalable process for hPSC-macrophage production (Ackermann et al. 2022) and the formation of definitive endoderm as well (Sahabian et al., 2021), underscoring the universal applicability of our strategy.
However, regarding the production of hPSC-CMs for cell therapies to the failing heart, we are working towards initiating a human trial. With this in mind, we must establish the GMP-compliant generation of the cell product considering all process steps, equipment, media, and materials as well as specific control steps to ensure constant cell quality.
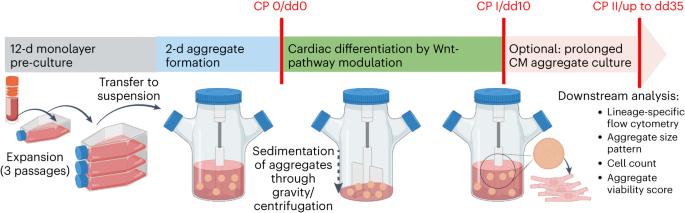
We start by cultivating hPSCs in conventional monolayer culture. This is already a potential pitfall, as the broadly applied growth matrix Geltrex required for 2D cultivation is a poorly defined substance, derived from a murine tumour cell line hindering GMP-compliance. However, we were happy to see that GMP-conform growth matrices such as CTS Vitronectin are compatible with our protocol. We then inoculate the spinner flasks with monolayer-derived single cells, to form stirring-controlled cell-only aggregates of relatively homogenous size in a two-day process in a pluripotency-supporting E8 medium. Directed differentiation is then induced via modulation of the Wnt pathway that is by cultivating the hPSC aggregates in medium containing CHIR99021 for 24 hours, followed by cultivation in medium containing IWP2 for 48 hours, as we have described earlier (Halloin et al., 2019).
After 10 days of differentiation, we assess the content of CMs in our suspension-generated cardiac aggregates. Our 300 ml process scale typically yields about 100 - 200 million cells at >90% CM purity shown by flow cytometry analysis specific to cardiac markers such as cardiac troponin T (cTnT), myosin heavy chain (MHC) and sarcomeric alpha-actinin (SA).
However, when considering the therapeutic application of these cells, investigating the identity of remaining non-CMs is important as well. The main risk factor in this regard are residual pluripotent cells, which, when transplanted into a patient, may develop into benign tumors known as teratomas. Importantly, we did not identify cells expressing typical pluripotency-related markers such as Oct3/4 in our cell population. Our comprehensive flow cytometry analysis revealed that the majority of non-cTnT cells express the cardiac progenitor marker NKX2.5, indicating that these cells will form functional CMs upon further maturation in vitro or in vivo i.e. post-transplantation. The other non-cTNT-expressing cells from our process express typical fibroblast-like connective tissue markers or markers of epicardial cells or neuroectodermal lineages.
An important logistic challenge for clinical applications is the distribution to the bedside since the long-term storage of cardiac aggregates, our favored cell product for transplantation, via the direct cryopreservation of whole aggregates is an unsolved challenge. In this regard, we have successfully demonstrated the extended cultivation of hPSC-CMs aggregates in suspension culture for an additional 25 days while maintaining cell phenotype, thereby enabling new options for downstream applications. Our strategy allows performing the various required quality control assessments before the cell product is clear for transfer to the bedside and transplantation.
Given the extensive practical workload required to complete our study, we would like to especially thank Mira, Jana, and Annika for their extensive high-quality support in all related cell culture work. This publication was kicked off by our efforts in drafting standard operating procedures (SOPs) for hPSC-CM production in our lab, driven by our former colleague Wiebke and myself. If you are interested in seeing how CMs that are produced according to this protocol perform in vivo after transplantation into damaged hearts in a non-human primate model, I recommend checking our preprints on bioRxiv.org (Gruh et al., 2024), revealing data generated by the project “iCARE” funded by the Federal Ministry of Education and Research.
We hope that our detailed protocol will provide many researchers with a relatively simple, yet efficient strategy for producing meaningful quantities of hPSC-CMs for their different applications such as cell therapies, tissue engineering, or drug discovery and safety assays.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in