Methane is an abundant and economically competitive carbon source for producing fuels and chemicals. However, due to the inert nature of methane, thermochemically converting it into high-value products typically requires high temperatures (> 700°C). Therefore, discovering methods to upgrade methane into value-added products under milder conditions remains one of the most significant challenges in sustainable and energy chemistry. The current industrial process necessitates initial steam reforming of methane at high temperatures (700-1000°C) to produce synthesis gas, which proceeds to produce methanol via Fisher-Tropsch synthesis at high pressure.1, 2 Approximately 65% of methanol production follows this route (Figure 1).3, 4 Methanol is further oxidised to HCHO over silver-based or iron-molybdate-based catalysts at temperatures around 350°C.5 More than 30% of methanol consumption results from HCHO production via this process.6, 7 Nevertheless, this multi-step process is inherently energy-intensive with huge CO2 emissions and only economically feasible on a large scale.
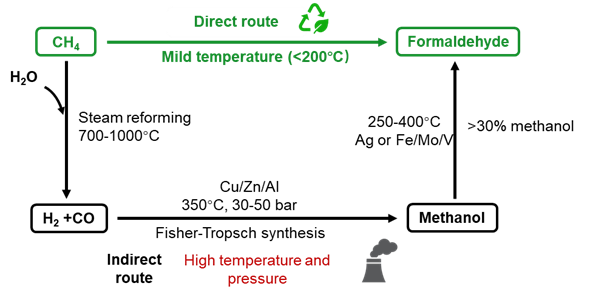 Figure 1. Proposed green and current industrial processes for HCHO production from methane.
Figure 1. Proposed green and current industrial processes for HCHO production from methane.
Here, we show a photon-phonon driven cascade reaction that allows for methane conversion to formaldehyde with an unprecedented productivity of 401.5 μmol h−1 or 40150 μmol g−1 h−1 and a high selectivity of 90.4% at 150℃ (Figure 2). Specifically, by a Ru single atom decorated ZnO catalyst, methane first reacts with water to selectively produce methyl hydroperoxide via photocatalysis, followed by a thermodecomposition step yielding formaldehyde. The present reaction route with minimised energy consumption and high efficiency suggests a promising pathway for the sustainable transformation of light alkanes.
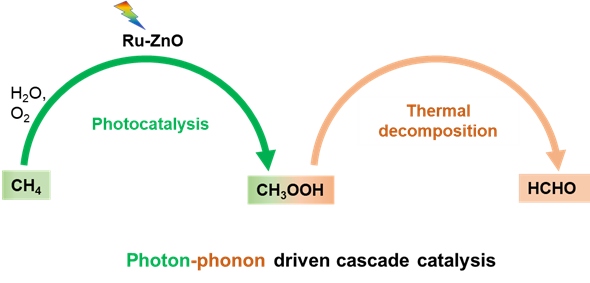 Figure 2. Photon-phonon driven cascade catalysis for methane oxidation to formaldehyde.
Figure 2. Photon-phonon driven cascade catalysis for methane oxidation to formaldehyde.
We initially focused solely on the photocatalysis of methane conversion. The primary product was CH₃OOH, which led us to ponder its potential applications. Through literature review and preliminary experiments, we realised that CH₃OOH is quite unstable and tends to decompose into methanol or formaldehyde by thermal catalysis, indicating that CH₃OOH can be an intermediate for the subsequent high-value chemical synthesis. We attempted to introduce thermal catalysis to promote photocatalysis for converting methane into stable end products. Our subsequent experiments reveal that phonon at 150°C could selectively decompose CH₃OOH into formaldehyde. Thus the concept of "photon-phonon driven cascade catalysis" has been achieved and successfully employed for methane to formaldehyde synthesis.
Our understanding of the reaction mechanism also evolved through a complex process. Initially, it was assumed that the oxygen in CH₃OOH originated from O₂. However, further experiments showed that the oxygen in HCHO came from H₂O. Based on extensive experimental results and theoretical calculations, it is finally proposed that methane reacts with water to selectively form the CH₃OOH intermediate, which then thermally decomposes into HCHO.
This journey has been both challenging and rewarding, leading to a deep understanding of the cascade process involving light and heating in catalysis. We hope that our findings will pave the way for further advancements in the field of methane conversion.
To learn more about our work, please read our article "Efficient methane oxidation to formaldehyde via photon–phonon cascade catalysis" in Nature Sustainability (https://www.nature.com/articles/s41893-024-01401-y).
Reference
- Sushkevich, V. L., Palagin, D., Ranocchiari, M. & Van Bokhoven, J. A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356, 523-527 (2017).
- Agarwal, N. et al. Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 358, 223-227 (2017).
- Dummer, N. F. et al. Methane Oxidation to Methanol. Chemical Reviews 123, 6359-6411 (2023).
- Agency, I. R. E. Innovation Outlook : Renewable Methanol. IRENA AND METHANOL INSTITUTE (2021).
- Bahmanpour, A. M., Hoadley, A. & Tanksale, A. Critical review and exergy analysis of formaldehyde production processes. Rev. Chem. Eng. 30, 583–604 (2014).
- Malik, M. I., Abatzoglou, N. & Achouri, I. E. Methanol to formaldehyde: An overview of surface studies and performance of an iron molybdate catalyst. Catalysts 11, 893 (2021).
- Wang, H. et al. High quantum efficiency of hydrogen production from methanol aqueous solution with PtCu–TiO2 photocatalysts. Nat. Mater. 22, 619–626 (2023).
Follow the Topic
-
Nature Sustainability

This journal publishes significant original research from a broad range of natural, social and engineering fields about sustainability, its policy dimensions and possible solutions.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in