In this paper a fixation and cleavage of the N≡N triple bond has been achieved in a dinitrogen (N2) matrix by the reaction of hydrogen and laser-ablated silicon atoms. The four-membered heterocycle H2Si(μ-N)2SiH2, the H2SiNN(H2) and HNSiNH complexes were characterized by infrared spectroscopy in conjunction with quantum-chemical calculations. The synergistic interaction of the two SiH2 moieties with N2 results in the formation of final product H2Si(μ-N)2SiH2, and theoretical calculations reveal the donation of electron density of Si to p* antibonding orbitals and the removal of electron density from the p bonding orbitals of N2, leading to cleave the non-polar and strong NN triple bond.
As every first semester student knows, nitrogen has one of the most stable bonds and it is therefore one of the standing challenges to activate and cleave this triple bond. Over more than 100 years, the industrial synthesis of NH3 has relied on the transition metal catalyzed Haber-Bosch (H-B) process in which the inert dinitrogen is converted to ammonia under harsh reaction conditions (450 degrees Celsius and 300 bar). Although the H-B process is essential to satisfy the food needs of the growing world population, its harsh reaction conditions, high CO2 emissions and large energy consumption are still widely concerned. Thus, converting dinitrogen to ammonia with less energy consumption is crucial for sustainable development.
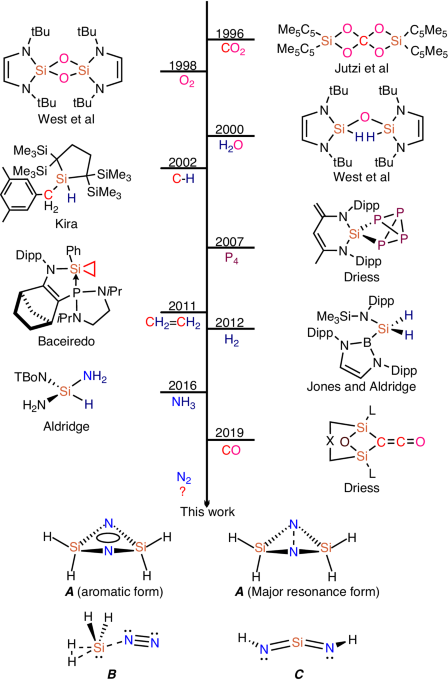
The N≡N triple bond activation is based on partially filled d-orbitals of the d-block elements (e.g. Fe) which have a suitable symmetry and energy. Given that transition metals can weaken or break the strong N≡N triple bond by donating electrons from their d orbitals into the p*-antibonding orbitals of N2, an alternative way to activate dinitrogen is through main group element compounds via the p back-donation from p orbitals (Be, B, C). Such an imitation occurs, for example with a non-bonding (donor) electron pair and an energetically low-lying vacant (acceptor) orbital. The p-block element boron was successfully used to mimic such a transition metal nitrogen activation. For example, our group has recently reported a complete cleavage of the N≡N triple bond by fluoroborylene (:BF) which has been observed as a cyclic FB(μ-N)2BF system in a matrix-isolation investigation under cryogenic conditions. Carbene, another reactive intermediate, has also been used for N2 activation and conversion. Based on these previous works it can be assumed that other p-block systems with suitable π back-bonding orbitals could also be used for N2 activation and conversion.
Silylene, the silicon analogue of carbene, has been proven to exhibit similar properties and even become an alternative to transition metal compounds, as it has a narrow HOMO-LUMO energy gap, which has received particular attention for the activation of small molecules. Many small stable molecules, such as CO2, O2, H2O, P4, C2H4, H2, NH3, and C-H bonds have been activated by silylenes in the previous research efforts. In 2019 the splitting and reductive homocoupling of CO was achieved using the bis-silylenes (LSi:)2Xant [Xant = 9,9-dimethylxanthene-4,5-diyl; L = PhC(NtBu)2] and (LSi:)2Fc (Fc = 1,10-ferrocenyl). Based on these results we speculated that, under the right circumstances, silylene could be able to coordinate and functionalize dinitrogen as well. The key difficulty in silylene-mediated nitrogen activation is to modify the occupied and vacant orbitals of silylene in space and energy, which could enhance the weakening and functionalization of an inert chemical bond.
In this work, we report on the activation of dinitrogen by silylenes under cryogenic conditions. In our matrix-isolation experiments, laser-ablated silicon atoms have been reacted with H2, D2, HD and H2/D2 mixtures in solid nitrogen that served as both reactant and host-matrix material. The activated dinitrogen specie H2Si(μ-N)2SiH2 has been identified by isotopic substitution experiments under cryogenic conditions in matrix-isolation experiments in conjunction with quantum-chemical calculations. The cyclic species H2Si(μ-N)2SiH2 is spectroscopically and quantum-chemically characterized, which demonstrates that the N≡N triple bond is cleaved by synergistic interaction of the two SiH2 moieties. Based on our experiments and quantum-chemical calculations, the SiNN is supposed to be an important starting compound that reacts with H2 molecules to form the complex H2SiNN(H2), which further reacts with SiNN through hydrogen transfer to give final complex H2Si(μ-N)2SiH2. Notice the N2 is only bound with one SiH2 in weakly end-on mode, but with two silylenes an NN triple bond is cleaved, that is to say, in our low temperature matrix silylene-mediated nitrogen activation occurs through the synergistic interaction of the two SiH2 moieties with N2
The H2Si(μ-N)2SiH2, complex is formed by two SiH2 subunits, in which the Si---Si distance in H2Si(μ-N)2SiH2 complex is well-controlled in low temperature matrix, finally leading to an N≡N triple bond cleavage. The EDA-NOCV calculations support a dual interaction of H2Si:, which is able to donate electron density to p*-antibonding orbitals of N2 (push effect) and remove electron density from the p-bonding orbitals of N2 (pull effect) and therefore cooperatively cleaves the N≡N triple bond and forms the stable aromatic ring system Si2N2 (Figure 1). This research work realized the activation of inert nitrogen by silylenes under cryogenic conditions. It might open a different way to functionalize and activate dinitrogen molecules which may contribute to a more fundamental design of catalysts based on silylenes for an artificial dinitrogen activation.
For more details on the experiments and results, please read our paper:
DOI: 10.1038/s41467-024-48064-z



Figure 1 Schematic diagram of the push and pull effects in H2
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Cannot find the actual paper... The DOI is invalid, and why not to include a direct link to a paper at the very top of the post?