Navigating a "target-rich" disease with liquid biopsies
Published in Cancer

Cholangiocarcinoma or bile duct cancers are increasingly being recognized. In United States, more than 40,000 new cases and 30,000 deaths in 2019 will be attributable to this disease.
One important advancement for patients with cholangiocarcinoma in the last few years has been the identification of potentially actionable genetic aberrations or findings. By actionable we mean that either they already have a new drug besides chemotherapy that could be used for these patients or that there are active ongoing open clinical trials for these patients that already are showing promise. Experts would often use the term "target-rich" since if we just glance at figure below, the odds of finding an actionable finding is pretty high.
Figure: Summary of increasing list of potentially actionable genetic aberrations or findings in patients with cholangiocarcinoma - a "target-rich" disease.
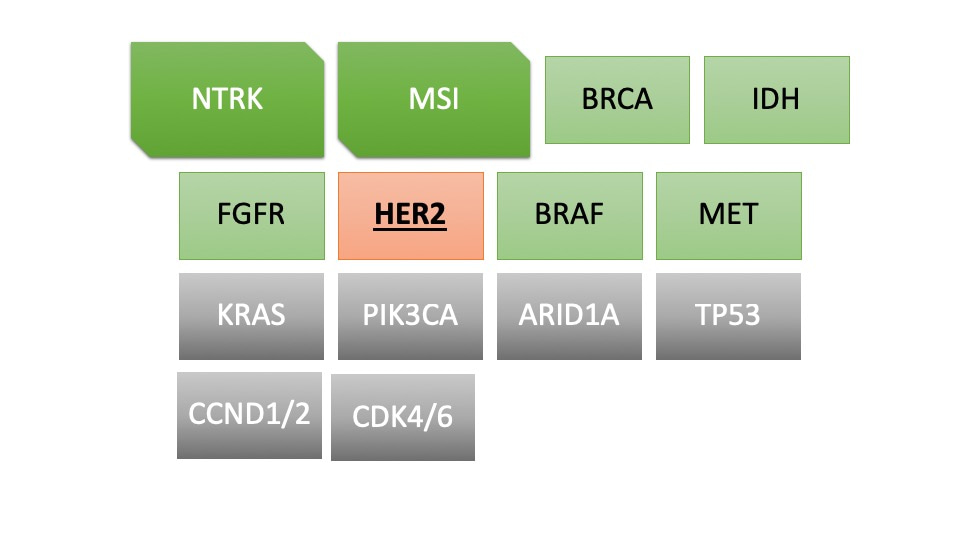
However, one barrier towards knowing this is often the lack of enough tissue for genetic testing. Diagnosis of cholangiocarcinoma is often challenging. It is not uncommon for patients to have undergone more than 1 endoscopic procedure (ERCP/EUS) to even get to a diagnosis. Tissue obtained often in these situations is not sufficient for further genomic testing. To subject a patient to another biopsy is also not safe or practical. Patients do not prefer that either.
This barrier could be overcome by employing "liquid biopsies" (circulating tumor DNA - ctDNA testing). We started using some of these commercially available platforms for our patients in the clinic serendipitously. However, we quickly realized that it indeed is of value particularly in the advanced/metastatic settings. Using these assays are of particular value in a patient with cholangiocarcinoma or any other cancer patient where the tissue is not in-house or if it is not safe or feasible to get more tissue. Results are available usually within 7-14 days and can alert the treating oncologist about predictive markers, which can then help guide treatment or chose clinical trials.
Herein we describe one such real clinical scenario, where if it was not for the liquid biopsy, we would have not known about the actionable finding (in this case HER2 +++ amplification), because of which anti-HER2 therapy was chosen. What is remarkable is that even as of today as the case is published, I was able to share this report with the patient and her family, who still continues to derive benefit from off-label anti-HER2 blockade. This part of the report is the most rewarding piece of it all.
Our report narrates as to how liquid biopsies are already having an impact in patients with cholangiocarcinoma. Additionally we also summarize the current literature on anti-HER2 therapy for cholangiocarcinoma. This would likely become another treatment option for this target-rich disease.
Follow the Topic
-
npj Precision Oncology

An international, peer-reviewed journal committed to publishing cutting-edge scientific research in all aspects of precision oncology from basic science to translational applications to clinical medicine.
Related Collections
With Collections, you can get published faster and increase your visibility.
Tumor-type-agnostic biomarkers and treatments in oncology
Publishing Model: Open Access
Deadline: Mar 05, 2026
Emerging adjuvant and neo-adjuvant treatment approaches in solid tumors
Publishing Model: Open Access
Deadline: Mar 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in