Non-Isocyanate Polyurethanes from Terpenes Using Thiourea Organocatalysis and Thiol-Ene-Chemistry
Published in Chemistry

Polyurethanes have many applications in our daily lives, but their production is based on fossil fuels and the use of toxic substances. Thus, within the last years, a growing field of research focused on the synthesis of so-called Non-Isocyanate Polyurethanes (NIPUs).
One possible strategy to obtain NIPUs is by the opening of cyclic carbonates with amines. Cyclic carbonates are for instance available from the insertion of CO2 into epoxides. Terpenes are possible precursors of cyclic carbonates (see Figure 1). Their use is attractive, as they occur as byproducts in some processes, but terpene-based cyclic carbonates suffer from low reactivity in the formation of NIPUs.
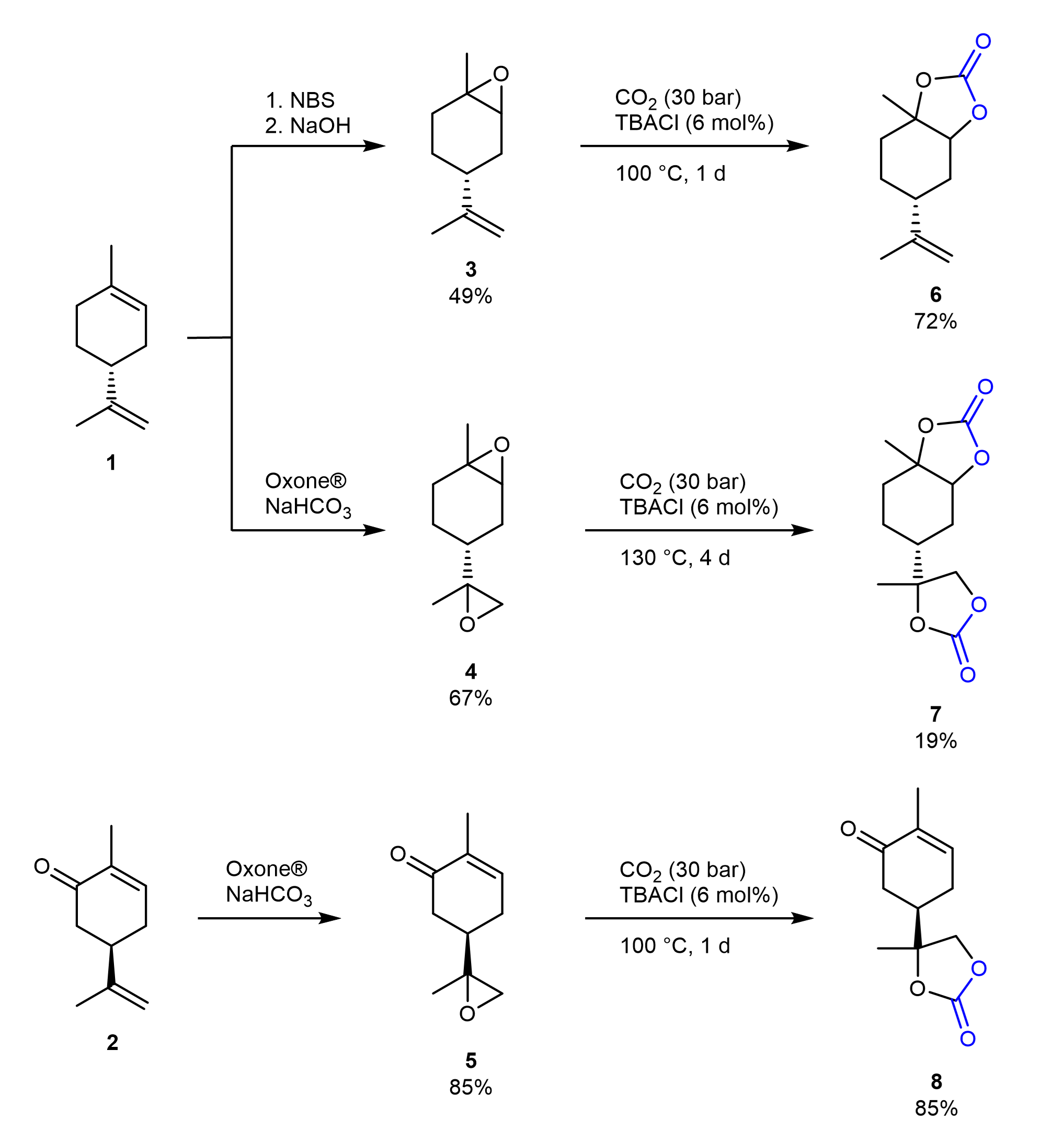
Figure 1. Synthesis of cyclic carbonates from limonene and carvone.
To increase the reactivity of terpene-based cyclic carbonates, organocatalysis can be used. Thiourea compounds (Figure 2), for instance, can activate the carbonyl center of cyclic carbonates and therefore facilitate their ring-opening with amines. In this work, we used thiourea organocatalysts to open terpene-based cyclic carbonates with amines containing terminal double bonds. Like this, AA urethane monomers are generated, which can be transformed into NIPUs by thiol-ene reaction with dithiols.
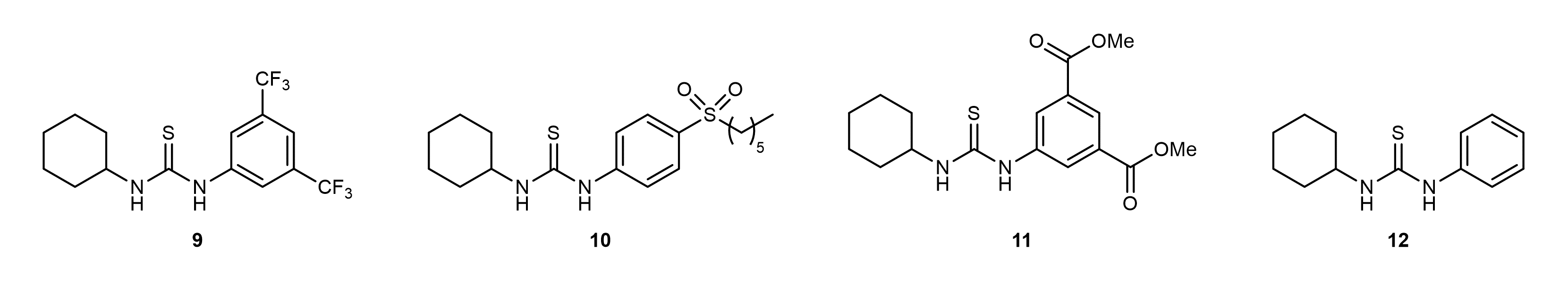
Figure 2. Thiourea organocatalysts used within this work.
The effect of the thiourea compounds on the opening of the cyclic carbonate groups was observed. Based on the starting terpenes limonene and carvone, two different types of cyclic carbonate groups can be distinguished: the exocyclic carbonate, which is not directly attached to the ring, does not need the addition of a thiourea compound to be opened by allyl amine. Contrarily, for the endocyclic carbonate group, in which the cyclic carbonate is directly attached to the terpene structure, the addition of catalytic amounts of an electron-poor thiourea significantly increased the conversion to the urethane monomer (Figure 3). It can be concluded that the cyclic carbonate unit interacts with the thiourea catalyst and the reaction proceeds faster.
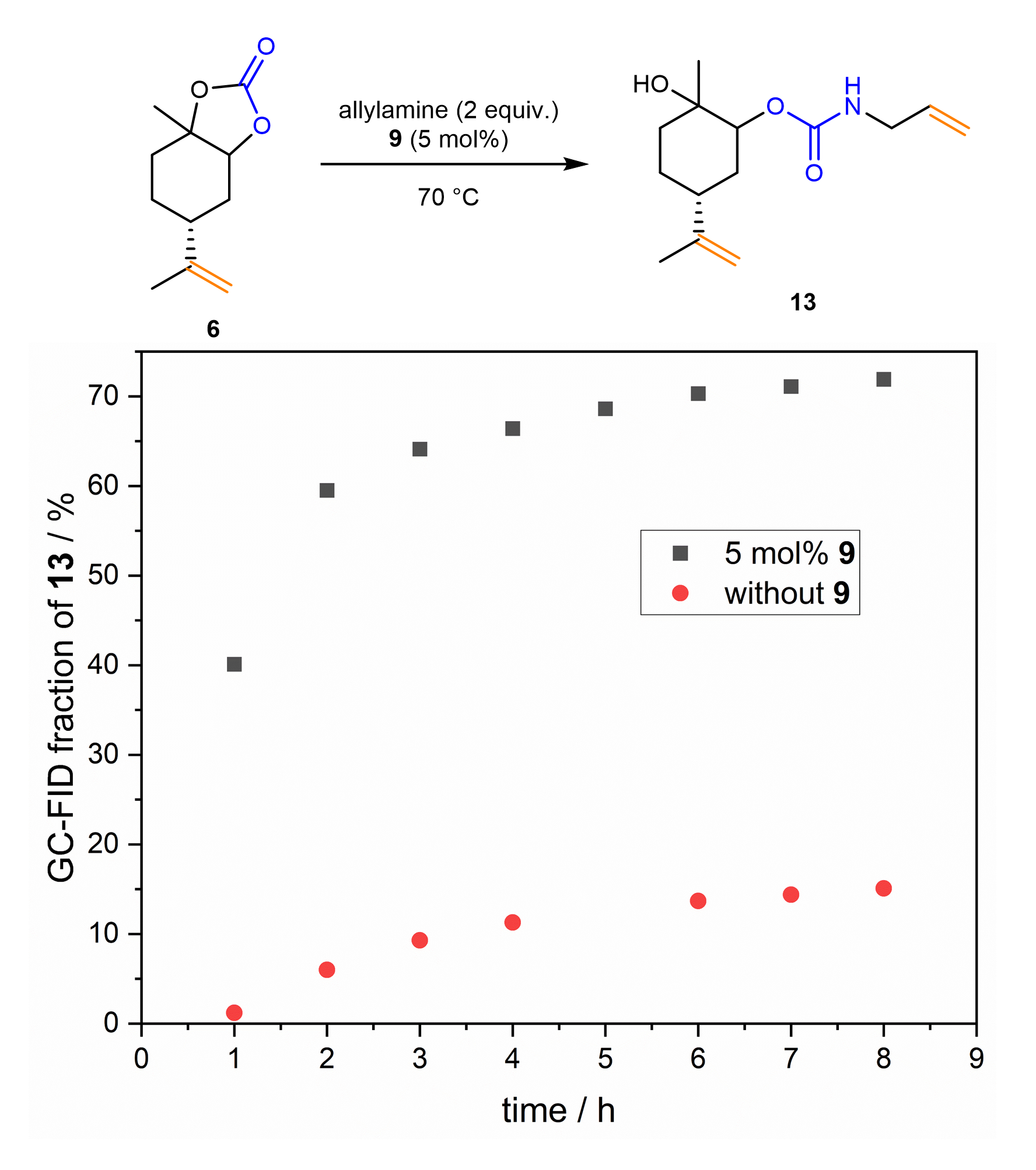
Figure 3. Conversion of limonene-derived carbonate to urethane monomer.
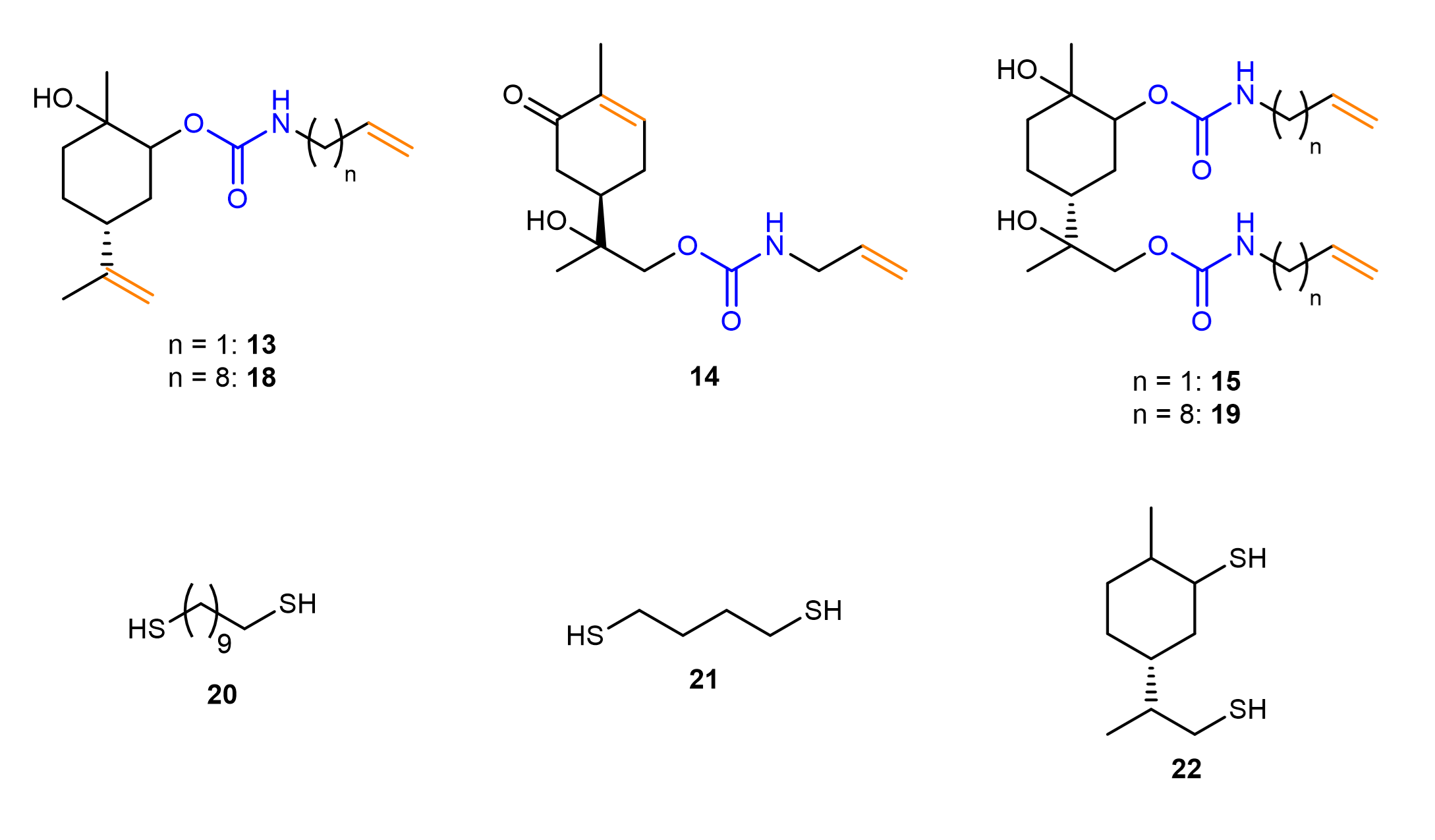
With this strategy, five different urethane monomers were synthesized by using different cyclic carbonates and two different amines, of which the longer one can be synthesized starting from castor oil derivatives. By reacting these monomers with different dithiols, linear NIPUs were prepared (see Table 1). Depending on the choice of monomer, different chain lengths are obtained. The analysis of the thermal properties shows glass transitions between 1 and 29°C. The difference in glass transition temperatures corresponds to the chain length of the amine, to the concentration of urethane groups and to the structure of the thiol.
Table 1. Variation of diene and dithiol within the synthesis of NIPUs. * Obtained from SEC of the crude reaction mixture, as precipitation of the polymer was not possible.
|
entry |
name |
diene |
dithiol |
Mn / kDa |
Ð |
Tg / °C |
|
1 |
P1 |
13 |
20 |
17.9 |
2.2 |
18 |
|
2 |
P2 |
13 |
21 |
15.1 |
1.9 |
16 |
|
3 |
P3 |
13 |
22 |
3.4* |
1.5* |
- |
|
4 |
P4 |
14 |
20 |
1.3* |
2.0* |
- |
|
5 |
P5 |
15 |
20 |
10.5* |
1.4* |
- |
|
6 |
P6 |
18 |
20 |
21.6 |
1.7 |
1 |
|
7 |
P7 |
18 |
22 |
6.1* |
1.5* |
- |
|
8 |
P8 |
19 |
20 |
31.2 |
1.8 |
23 |
|
9 |
P9 |
19 |
22 |
12.8 |
1.7 |
29 |
With this strategy, we can increase the accessibility of terpene moieties for the synthesis of polyurethanes. The polymers that we obtained are structurally different from polyurethanes that are used in our daily life, thus opening both challenges and potential for further research.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Advances in Asymmetric Catalysis for Organic Chemistry
Publishing Model: Open Access
Deadline: Mar 31, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in