Optogenetic Stimulation of Mouse Hoxb8 Microglia in Specific Regions of the Brain Induces Anxiety, Grooming, or Both.
Published in Neuroscience

Why Sweat It:
In this blog I will describe a paper which we wrote, Naveen Nagarajan and myself, Mario Capecchi, that was accepted for publication in Molecular Psychiatry, a very high-profile journal. Why might you find this paper interesting? It is about a very topical topic, “Anxiety”. Because of the Covid pandemic, the levels of anxiety have been rising enormously, throughout the world. A small amount of anxiety is good. Anxiety motivates us, spurs us on and gives us that extra bit of push that says I can. But a large dose of anxiety overwhelms us, we become mentally paralyzed, the heart beats faster, we sweat and confusion settles in our minds. That’s too much anxiety and that is our topic.
It started with making a mouse knockout in the Hoxb8 gene. The mice show both chronic anxiety and Obsessive-Compulsive Spectrum Disorder (OCSD) like behavior. This was quite unexpected because Hox genes are normally involved in assuring that all the components of our body are in the right place (body plan). That caught my attention, there must be a story here. The OCSD-like behavior in mice reflects a subtype of OCSD called trichotillomania which in mice results in hair removal as a consequence of pathological overgrooming to the extent of generating lesions at the sites of overgrooming. The next step was to determine which defective cells in Hoxb8 mutant mice were causative for the chronic anxiety and OCSD-like behavior. Another surprise, it was microglia and not neurons. Microglia are the immune cells of the brain which confront pathogens and clear cellular debris. We suggest that microglia also control neural circuits responsible for generating anxiety and normal grooming behavior and when microglia are defective, chronic anxiety and pathological overgrooming takes over. Not just any microglia, but a specific subpopulation of microglia, which we call Hoxb8 microglia because they are specifically marked by the Hoxb8 gene during development.
Based on elegant cell lineage tracing by F. Ginhoux et al., it was believed that there was only a single source of microglia, the yolk sac, starting at gestation day 7.5, followed by migration of the microglia progenitor cells into the developing brain at E9.5. However, by their own admission, Runx1 their cell lineage marker, could only account for ~60% of adult microglia, leaving room for additional microglial cell lineages. Subsequently, our laboratory demonstrated that there are at least two progenitor pools for microglia: canonical non-Hoxb8 microglia and Hoxb8 microglia that are also born in the yolk sac but a day later (E8.5) and then migrate to the AGM (Aorta Gonad Mesonephros region) and fetal liver where they are amplified 16 and 280-fold respectively, prior to their entry into the developing brain, starting at E12.5. Though non-Hoxb8 microglia comprise ~70% of total microglia in the adult brain, they cannot compensate for the loss of Hoxb8 microglia function (~ 30% of microglia in the adult mouse brain) arguing for the separation of functions between these two subpopulations of microglia. We went on to demonstrate that defective Hoxb8 microglia are causative for both behavioral pathologies.
That brings us to the present. We needed a means to connect Hoxb8 microglia to the neurons/neuronal circuits that regulate anxiety and grooming behaviors. A candidate technology was optogenetics, the selective use of light activated cation channels to stimulate microglia. This may not appear to be a good choice for this purpose since microglia are not neurons and cannot generate action potentials. However, optogenetic stimulation of microglia should minimally depolarize microglial cell membrane potential, that in turn could cause microglia to send signals to neighboring neurons and activate them. Specific optogenetic stimulation of Hoxb8 microglia in chosen regions of the brain worked brilliantly. In live mice using optic cables to specifically stimulate Channelrhodopsin2 (ChR2) using blue laser light, a light activated ion channel, in Hoxb8 microglia within chosen regions of the brain resulted in inducing anxiety, grooming or both dependent upon which regions of the brain were chosen for laser activation! Turn on the laser and the behavior starts, turn off the laser and the behavior stops. Now we have the means to determine which neurons in each region are Hoxb8 microglia communicating with and ability to determine the molecular mechanisms for the communication between microglia and neurons.
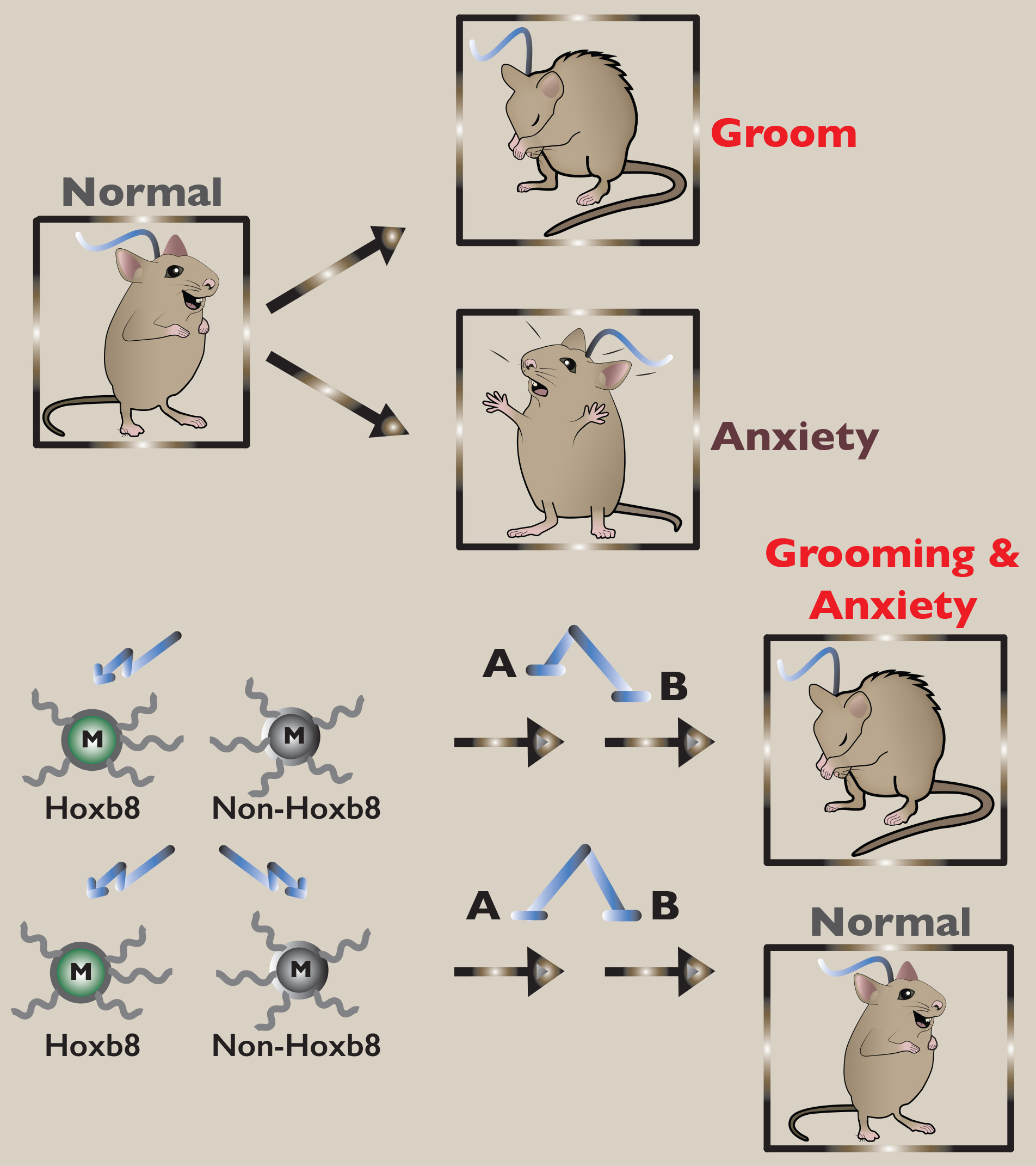
Thus far we have discussed the behavioral consequences of optogenetic stimulation of Hoxb8 microglia in the different regions of the brain. What are the behavioral consequences of stimulating non-Hoxb8 canonical microglia in the same regions of the brain? Unfortunately, we cannot directly do those experiments because to date a genetic marker specific to non-Hoxb8 canonical microglia does not exist. However, we can optogenetically stimulate both microglia subpopulations and compare the results with optogenetic stimulation of Hoxb8 microglia alone. Our expectation for these experiments was that either 1) no behavioral differences would be observed between activation of both microglia populations and activation of only Hoxb8 microglia, indicating that non-Hoxb8 microglia do not participate in the induction of grooming or anxiety or 2) higher levels of grooming and anxiety would be observed by activating both microglia populations compared to activation of only Hoxb8 microglia, suggesting that both microglial populations participate in the induction of the behaviors.
Instead the experimental results were totally unexpected, no behaviors were induced! Optogenetic stimulation of both microglial subpopulations in the appropriate regions of the brain resulted in quenching the behaviors induced by stimulation of Hoxb8 microglia alone. These results required us to completely rethink on how the two populations of microglia function with each other to regulate grooming and anxiety. With respect to these functions, the two populations of microglia must be working in opposition to each other. For example, Hoxb8 microglia function to down regulate the behaviors (i.e. function as brakes) whereas non-Hoxb8 microglia are upregulating the behaviors (i.e. functioning as accelerators). Together they set the appropriate level of behavioral output. Further, the model is consistent with all the results that we have obtained by genetic manipulations of Hoxb8 microglia. Most importantly, the model provides a biological reason for the existence of two microglia subpopulations in mice! We provided direct evidence that optogentic stimulation of Hoxb8 microglia results in activation of neuronal activity (spiking activity) in neighboring neurons. Further, optogentic stimulation of Hoxb8 microglia in vivo leads to upregulation of c-fos in neighboring neurons. Finally, we have provided independent data that supports our model that the two microglial subpopulations work in opposition to each other with respect to regulating the levels of anxiety and grooming. Now both populations of microglia become targets for the development of drugs to control chronic anxiety or OCSD. Meanwhile, we will be working on the molecular mechanism of how these two populations of microglia control to maintain normal grooming and anxiety behavior and how mismanagement of the levels of these behaviors results in pathological behaviors, chronic anxiety and OCSD-like behavior.
Follow the Topic
-
Molecular Psychiatry

This journal publishes work aimed at elucidating biological mechanisms underlying psychiatric disorders and their treatment, with emphasis on studies at the interface of pre-clinical and clinical research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcement



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in