Preliminary Phase 2a Readout for a Novel Drug Discovered and Designed Using Generative AI Sets a Major Milestone in AI-Powered Drug Discovery
Published in Social Sciences, Chemistry, and Computational Sciences
A Small Molecule TNIK Inhibitor Identified and Designed by Generative AI
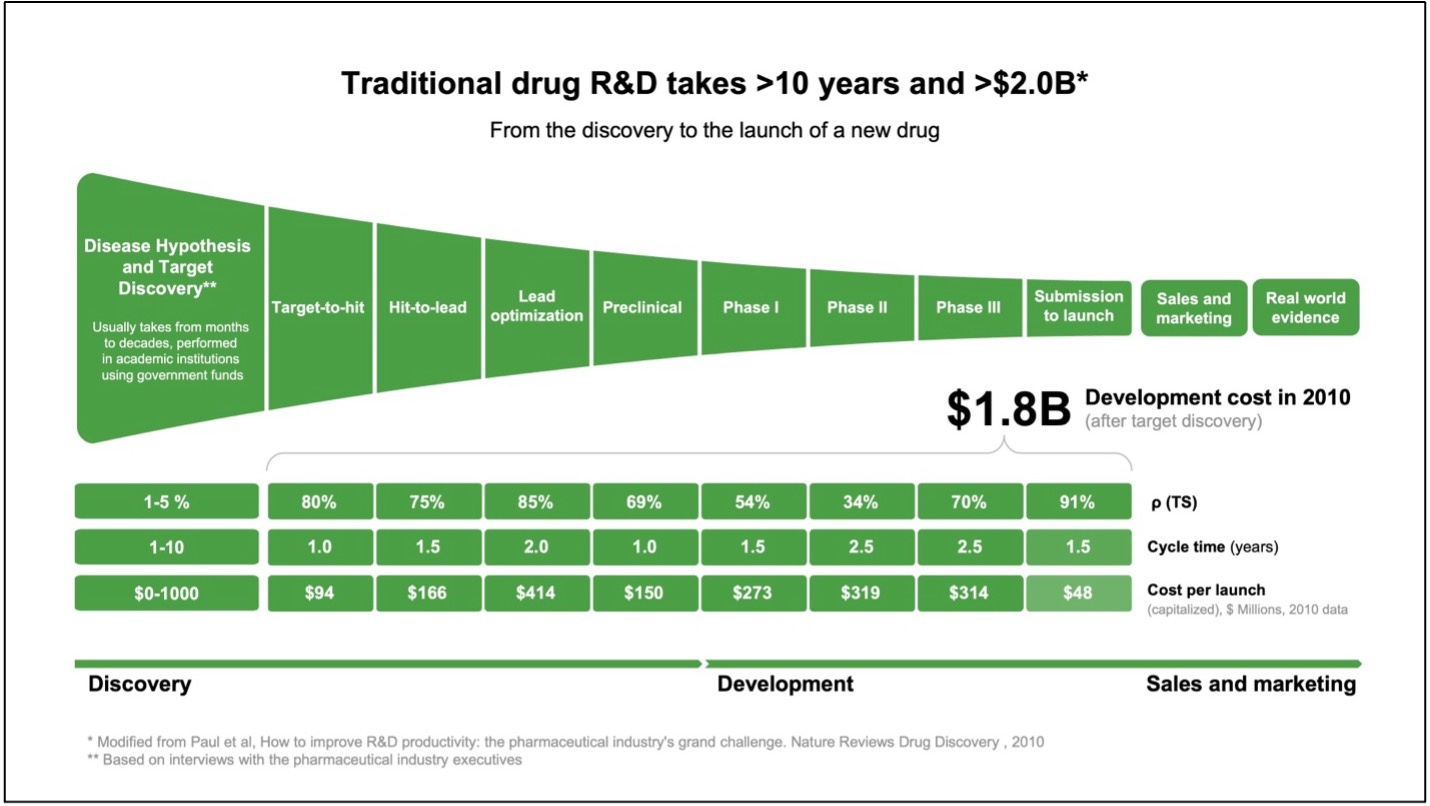
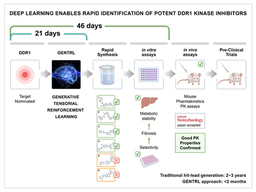
The traditional drug discovery process is a time-consuming, financially demanding, and risk-filled endeavor that often takes more than 10 years to complete and costs more than>$2.0B. From its inception, Insilico Medicine’s goal has been to use advanced generative artificial intelligence (AI)-powered approaches to streamline and improve this pipeline, expediting the rate at which novel therapies are safely brought into the clinic. One of the Company’s flagship programs has shown immense potential in treating idiopathic pulmonary fibrosis (IPF), a debilitating and age-related lung disease that lacks a curative treatment. ISM001-055 is a novel, generative AI-designed small molecule inhibitor that targets TNIK (TRAF2 and NCK-interacting kinase), a protein kinase that orchestrates several pro-fibrotic pathways that drive IPF pathology.
From Hypothesis to Phase 1 Clinical Success in Less than 30 Months
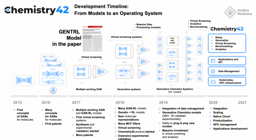
The generative artificial intelligence platform Pharma.AI is at the center of ISM001-055’s history. TNIK was first identified and prioritized as a target for treating IPF in 2019 using the Biology AI module of the Pharma.AI platform. Within a year, the Chemistry AI module aided our medicinal chemists and biologists in synthesizing ISM001-055. Our group rigorously tested ISM001-055’s safety, toxicity, and efficacy resulting in its preclinical candidate nomination in February 2021, just 18 months following its initial identification by Pharma.AI. This rapid timeline is a testament to the power of generative AI in streamlining the drug discovery process.
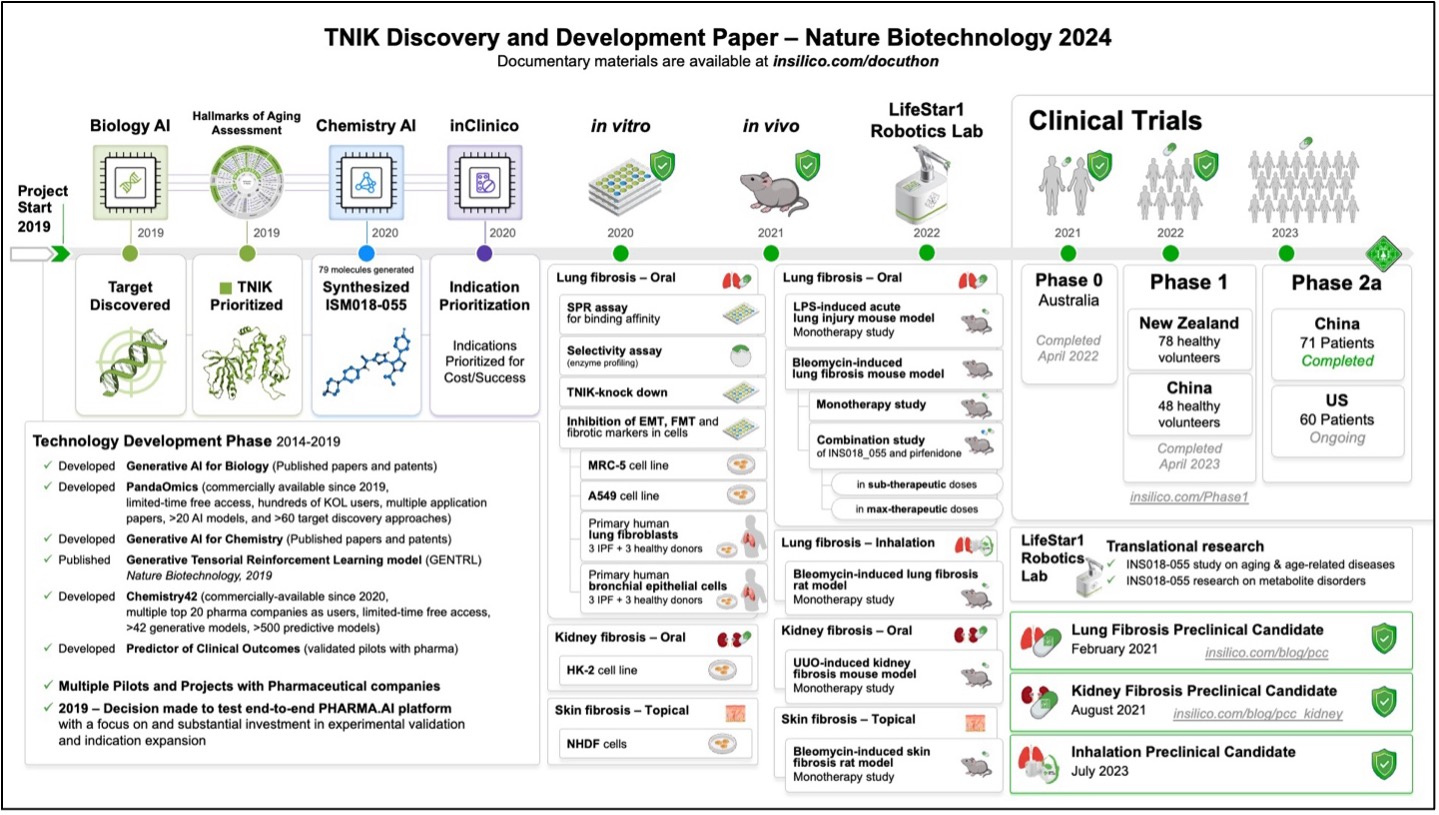
Following its preclinical candidate nomination, ISM001-055 was tested in multiple preclinical animal models of lung, kidney, and skin fibrosis. ISM001-055 exhibited favorable safety, favorable toxicity, and robust efficacy across all tested models justifying its in-human clinical testing beginning in 2021. After completing these investigational new drug-enabling studies, Insilico Medicine carried out a Phase 0 microdose trial and two, independent Phase 1 clinical trials in New Zealand and China to translate these studies into humans. Indeed, ISM001-055 was found to be safe, and well-tolerated by volunteers across all 3 trials. Investigators observed no significant accumulation after 7 days and ISM001_055 exhibited a favorable pharmacokinetic profile. In March 2024, this collective body of work from target identification to Phase 0/1 clinical trial completion was peer-reviewed and published in Nature Biotechnology as ISM001-055 progressed into Phase 2 clinical testing.
Favorable Topline Results for Phase 2 Clinical Trial

In this 12-week study, ISM001-055 met its primary endpoint of safety and tolerability across all dose levels. The secondary efficacy endpoints were also met with ISM001-055 demonstrating a dose-dependent improvement in forced vital capacity (FVC), a critical lung function measure in IPF patients. Patients receiving 60 mg QD of ISM001-055 exhibited the largest improvement in FVC. The complete topline data will be released at an upcoming medical conference with clinical trial results to be submitted for publication in a peer-reviewed journal. A separate Phase IIa(NCT05975983) clinical trial in the U.S. is ongoing and actively enrolling patients.
"These results are very encouraging, particularly the dose-dependent response in FVC. IPF is a devastating disease, and seeing improvements in lung function over just 12 weeks of treatment is a promising indication that ISM001-055 may provide a new therapeutic option for patients. Our Phase IIa in the U.S. is actively recruiting patients," said Toby M. Maher, MD, PhD, a leading expert in interstitial lung disease and an investigator in the trial.
“This study result represents a critical milestone in AI-powered drug discovery and in my life to date,” said Alex Zhavoronkov, PhD, co-CEO of Insilico Medicine. “While we expected the drug to be safe, we did not expect to see such a clear dose-dependent efficacy signal after such a short dosing period. IPF is a very diverse disease and it is very rare to see improvement in FVC. With our novel TNIK inhibitor, we attempted to go after what we think is a common mechanism in fibrotic diseases and in aging to maximize indication expansion potential.”
“I am excited to see that ISM001-055 demonstrated obvious clinical efficacy in IPF patients in only 3 months of treatment. While preliminary, this clinical data is certainly encouraging, and provides the clinical validation of AI-powered drug R&D for both novel target and novel molecule,” said Feng Ren, PhD, co-CEO and CSO of Insilico Medicine. “ This is a significant milestone for Insilico Medicine and the AI-driven drug discovery Industry. The milestone is achieved due to the contribution of both the capabilities of our proprietary generative AI platform and the efforts of our multidisciplinary R&D team. We will continue to fully commit to providing breakthrough solutions for the benefit of the patients globally.”
Follow the Topic
-
Nature Biotechnology

A monthly journal covering the science and business of biotechnology, with new concepts in technology/methodology of relevance to the biological, biomedical, agricultural and environmental sciences.
Your space to connect: The Primary immunodeficiency disorders Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine, Immunology, and Diseases!
Continue reading announcement
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in