Using nanotechnology to improve cancer therapy
Published in Bioengineering & Biotechnology
Nanomedicine is a promising and rapidly emerging branch of nanotechnology, that is mainly focused on the targeted delivery of drugs for numerous types of diseases. Over past decades, it has gained special attention in oncology research, where various nanoparticles are used as nanocarriers transporting different anti-cancer drugs that allow selective targeting, improved drug efficacy, lower systemic toxicity, improved cellular uptake, and higher accumulation of the active reagents inside the tumor. While some of the nanocarriers have demonstrated promising preclinical results, few have entered clinical trials and none have been approved for treatment of human cancers.
Cisplatin (cis-(diamine) dichloroplatinum(II), CDDP), the first platinum-based anti-cancer drug approved by the US Food and Drug Administration (FDA), is a potent chemotherapy medication used to treat a number of solid malignancies including breast, ovarian, bladder, cervical, prostate, endometrial, and head and neck cancers. This frequently administered systemic chemotherapeutic agent acts primarily by forming DNA crosslinks, which prevents DNA replication and induces apoptosis, a process defined as “programmed” cell death. However, systemic administration of cisplatin intravenously results in non-selective targeting of both healthy and malignant tissues, provoking substantial adverse effects and toxicities including protracted nausea, vomiting, ototoxicity, acute nephrotoxicity, myelosuppression, and chronic neurotoxicity. These side effects of systemic cisplatin administration can be dose-limiting, reducing the dosage and length of treatment for patients, and subsequently limiting therapeutic benefits. Thus, finding novel therapeutic approaches for tumor-specific, controlled delivery is of the utmost importance to improve the efficacy of chemotherapy drugs and enhance health outcomes and the survival of patients.
To this end, a wide range of delivery systems are being investigated as alternative approaches for targeted administration of the anticancer agents. Two major methods, nanoparticle (NP) encapsulation of the active compound and topical administration, have been gathering attention as alternatives to the more traditional oral or intravenous delivery of the free drugs. The first NP-based treatment was approved by the FDA in 1995, and the field has grown exponentially ever since. Encapsulating chemotherapeutic agents, such as cisplatin, into the NPs (nano-sized, spherical, polymeric, synthetic particles) may increase their circulation time, improve deposition and concentrated retention of the drug at the tumor site, and subsequently enhance overall therapeutic efficacy. On the other hand, topical delivery of the nanoparticles in the form of gels and film composites has also been widely investigated for local administration of chemotherapeutic drugs, vitamins, peptides, and antibiotics to anatomically accessible cancers, such as oral cavity squamous cell carcinoma (OCSCC) - the most common type of head and neck cancer. Topical NP-based drug delivery platforms primarily intend to ameliorate the adverse effects of systemically administered treatment and to maximize the total dose and retention of the carried therapeutic agent at the local site, thus improving the treatment efficacy.
Currently, a wide range of nanoengineered, biocompatible, topical nanomedicines are being developed for advanced drug delivery applications in oncology and other medical areas. Among the most commonly used NPs for topical drug delivery are polymeric nanoparticles, nano-emulsions, lipid-based (liposomes and solid-lipid) nanoparticles, metal nanoparticles, and dendrimers. While some of these formulations, including Nanoplatin™, Aroplatin™, Lipoplatin™, and SPI-077, have demonstrated promising preclinical results and a few have entered clinical trials, none have been approved for the treatment of human cancers.
NP formulations based on chitosan, a non-toxic, biocompatible, and biodegradable polysaccharide derived from natural chitin through partial deacetylation, can be used for the delivery of active ingredients, such as drugs or natural products, by diverse routes of administration including oral and parenteral delivery. Chitosan nanoparticles are also particularly suitable for local administration at the dermis and the mucosa, owing to chitosan’s mucoadhesive and permeation-enhancing properties. While chitosan-based NPs have become of great interest in nanomedicine, biomedical engineering, and the development of new oncology drug release systems, none have been approved for treatment of solid malignancies, and only a few studies have tested the potential utilization of these approaches in oral cancer. The difficulty of maintaining the delicate balance of extracellular stability and intracellular drug uptake remains a major barrier that hinders the application of polymeric cisplatin-loaded NPs in oncology.
A new study, recently published in Nature Communications, is now revolutionizing this field through the development of PRV111, a nanotechnology-based transmucosal system designed to locally deliver cisplatin-loaded chitosan NPs (CLPs) directly to accessible oral cancers. As the authors put it “PRV111 is a thin, two-layered, matrix-type, polymeric transmucosal patch, consisting of a chitosan matrix layer embedded with CLPs and an impermeable ethyl-cellulose adhesive backing, intended to provide targeted drug delivery and prevent CLP washout from saliva”. In this platform, chitosan is used as a polymer for both the NPs and the porous matrix. As such, matrix-based water-soluble chitosan acts as a bioadhesive, since the positively-charged chitosan can bind to negatively-charged mucoproteins, allowing the electrostatic interaction with mucin proteins in the oral cavity. In technical terms, each PRV111 topical patch contains 0.5 mg/cm2 (2 mg in total) of cisplatin, covers a tumor region of 4 cm2, and incorporates a permeation enhancer that allows optimal penetration and absorption of the CLPs released from the patch. Notably, the released CLPs swell to approximately 0.5 micron when exposed to moisture, allowing them to diffuse across the porous matrix into the tumor tissue and regional lymph nodes. These particles are too large to penetrate into the vasculature, and therefore prevent systemic cisplatin exposure.
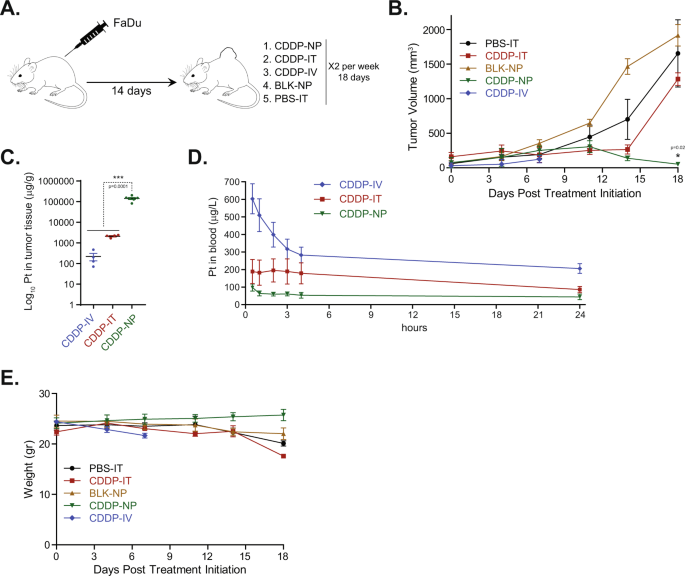
Through a series of multiple in vivo studies and a phase I/II in-human clinical trial, the authors of this manuscript demonstrate the efficacy of local administration of PRV111. PRV111 induced a robust anti-tumor response, reduced cisplatin-associated toxic adverse effects, and prevented tumor recurrence in preclinical models of this difficult-to-treat disease. In clinical trial settings, PRV111 promoted local retention of the cytotoxic drug (cisplatin) and induced a rapid anti-tumor response in patients with locally advanced OCSCC. Astoundingly, 87% of patients responded to treatment and showed decreased tumor volume, with an average decrease of 70% of tumor volume across all patients. Histological staining after surgery was further encouraging -- treatment with PRV111 showed at minimum a 10x increase in the number of tumor-infiltrating lymphocytes (TILs) present. Importantly, the TILs stained for were CD3, CD4, and CD8, which are all associated with increased survival in patients with oral cancer.
In principle, this novel nanoengineered mucoadhesive delivery platform can be engineered to deliver virtually any chemotherapy agent and other drug classes with a particular drug release profile and the desired dosage form, making it customizable for specific clinical applications. The unique properties of this carefully designed nanomedicine provides a promising framework and holds potential for the improved treatment of not only for OCSCC, but also other mucosal and skin malignancies. A second lead derivative of the PRV platform, PRV211, was designed for rapid drug release and is intended to be used in the operating room. PRV211 can be used on all solid tumor surgeries and can be placed on the tumor bed post-resection to eliminate residual cancer cells, treat nearby lymph nodes and ultimately reduce tumor recurrence.
If you would like to learn more about the paper, please read the interview with the co-authors in my recent Forbes.com post: "Going Beyond Target Or Mechanism Of Disease: Disruptive Innovation In Drug Delivery Systems".

Reference:
Goldberg, M., Manzi, A., Conway, P. et al. A nanoengineered topical transmucosal cisplatin delivery system induces anti-tumor response in animal models and patients with oral cancer. Nat Commun 13, 4829 (2022). https://doi.org/10.1038/s41467-022-31859-3
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in