PTEN-restoration abrogates brain colonisation and perivascular niche invasion by melanoma cells
Published in Cancer

Melanoma brain metastases (MBM) continue to be a significant clinical challenge and a marker of poor prognosis. Current therapeutics are less effective in controlling intracranial lesions compared to extracranial metastases. Pre-clinical studies have demonstrated that melanoma cells require contact with the vasculature to form brain metastases. Furthermore, melanoma cells are predominantly found within the perivascular niche of human patient MBM samples. However, while vascular co-option has been recognized to promote colonisation of the brain by invasive melanoma cells, the molecular mechanisms that drive melanoma:endothelial cell heterotypic interactions are lacking (1).
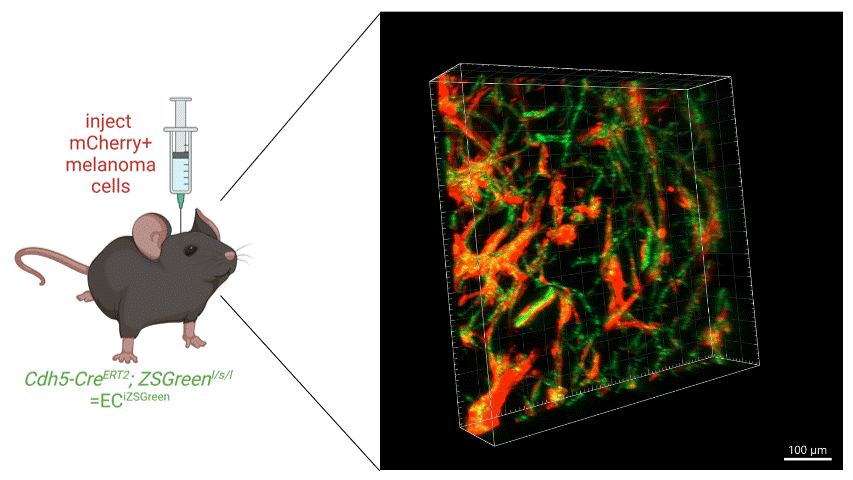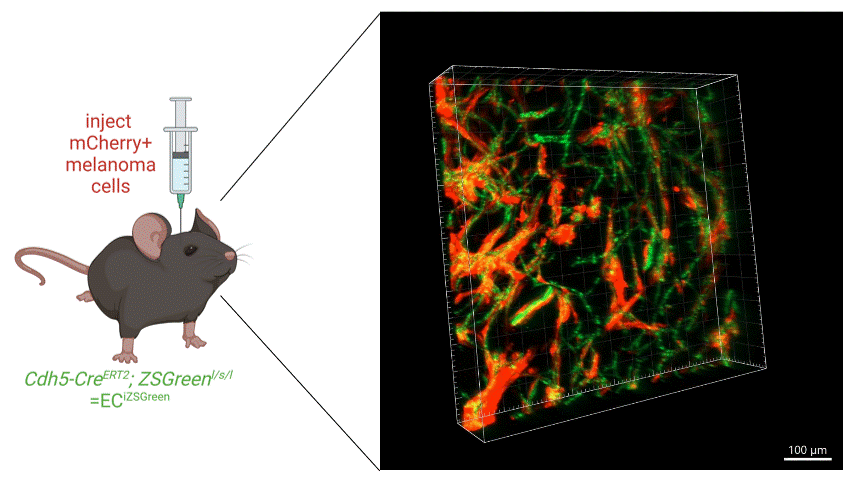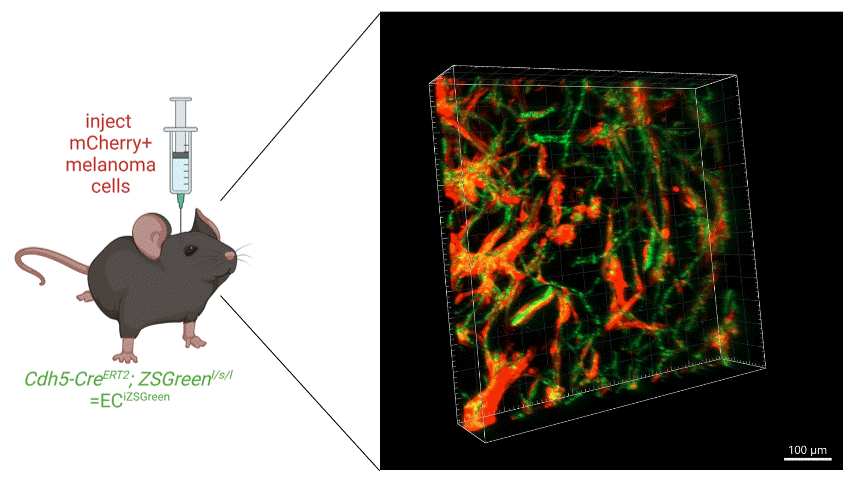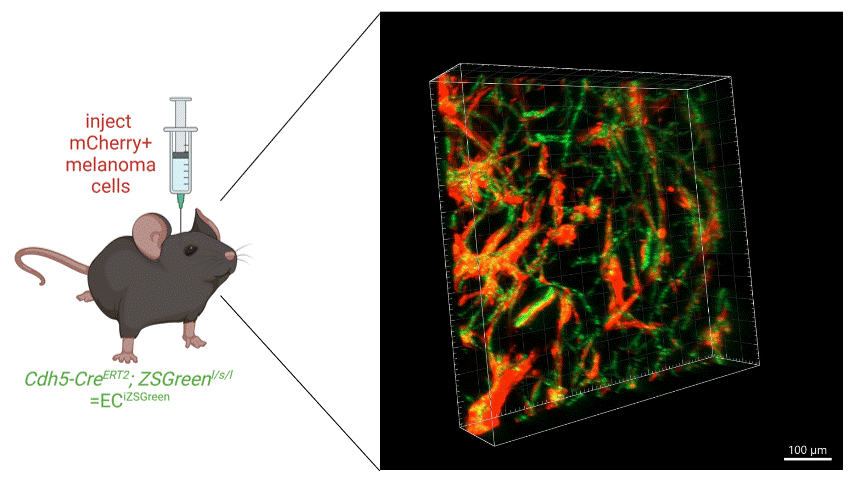
We intracranially injected mCherry-labelled melanoma cells into mice with ZSGreen-labelled vasculature and found a striking colocalization of cancer cells along the brain vasculature in a PTEN-null melanoma line, suggesting that these cells were competent at colonising the perivascular niche (see image below). Additionally, these cells formed more perivascular tumor clusters that were able to invade further from the invasive front of the tumor compared to either a PTEN-expressing melanoma cell line or to cells with re-expression of PTEN (PTEN-RE).
Bulk RNA sequencing of melanoma cells that were co-cultured with brain endothelial cells further demonstrated that the PTEN-null melanoma cells upregulated pathways indicative of thriving cells within the perivascular niche (e.g. PI3K/AKT signaling, ribosomal biogenesis), while PTEN-expressing melanoma cells upregulated pathways related to stress/inflammation upon contact with endothelial cells (e.g. apoptosis, fluid shear stress, TNF signaling, AGE-RAGE signaling). While both melanoma lines upregulated adhesion/migration pathways upon co-culture (e.g. ECM-receptor interactions, axon guidance, RAP1 signaling), the co-cultured PTEN-null melanoma cells had a further enrichment of an epithelial-mesenchymal transition (EMT) signature including several TGFβ-related genes compared to co-cultured PTEN-expressing melanoma cells.
To investigate the role of TGFβ, we used CRISPR to knockout the receptor TGFβR2 in melanoma cells; and found that disrupting melanoma response to TGFβ resulted in a reduction in brain colonisation and perivascular invasion in the in vivo intracranial model. To test whether this was mediated through a TGFβ-AKT axis, we re-introduced a constitutively-active form of AKT (myrAKT) into the TGFβR2 KO cells and found that myrAKT restored overall tumor size but not perivascular invasion. Thus, TGFβ-AKT signaling is important for brain colonisation of melanoma cells, whereas TGFβ-AKT independent mechanisms contribute to perivascular invasion (graphical summary below).
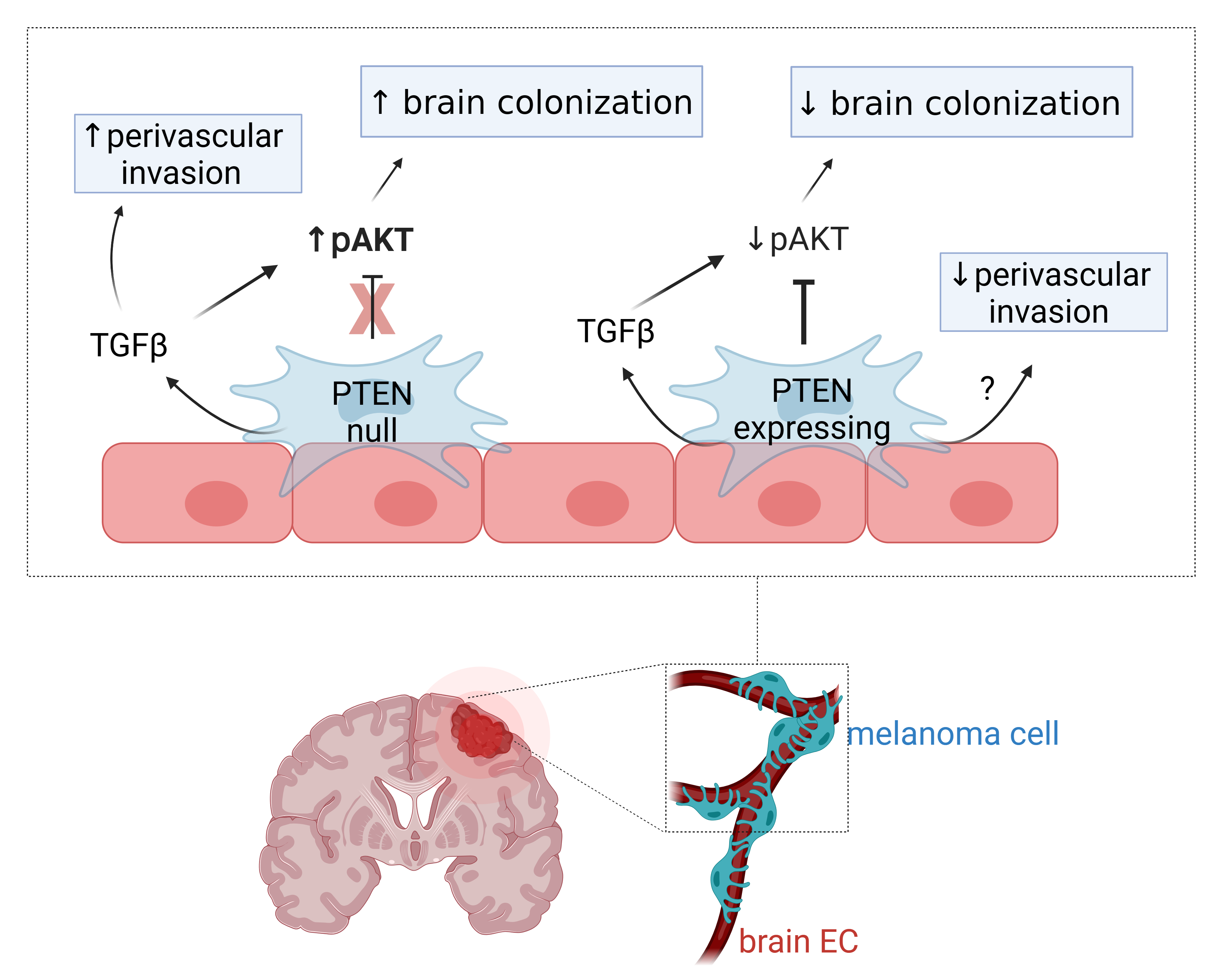
Taken together, our study highlights that the perivascular niche can serve as a highway for invasion of melanoma cells to disperse throughout the brain parenchyma and that vascular-mediated factors can provide important survival signals to cancer cells. We found that PTEN-null melanoma had a preference for co-opting the vasculature in comparison to PTEN-expressing counterparts and these melanoma cells are able to respond to perivascular TGFβ in order to survive within this microenvironment. This work described a new role for PTEN and TGFβ in perivascular invasion by MBM, though further studies are needed to dissect how these complex signaling networks ultimately drive adhesion to and movement along the vasculature. This is an important area of interest because interrupting cohesive interactions between melanoma cells and endothelial cells or depleting response to vascular-mediated signaling may represent a novel therapeutic strategy to treat MBM.
Dudley Lab - Tumor microenvironment and angiogenesis - (virginia.edu)
References:
(1) Zhang Y, Wang S, Dudley AC. Models and molecular mechanisms of blood vessel co-option by cancer cells. Angiogenesis. 2020;23(1):17-25.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.
Ask the Editor – Inflammation, Metastasis, Cancer Microenvironment and Tumour Immunology
Got a question for the editor about inflammation, metastasis, or tumour immunology? Ask it here!
Continue reading announcement




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in