PTMGPT2: An Interpretable Protein Language Model for Enhanced Post-Translational Modification Prediction
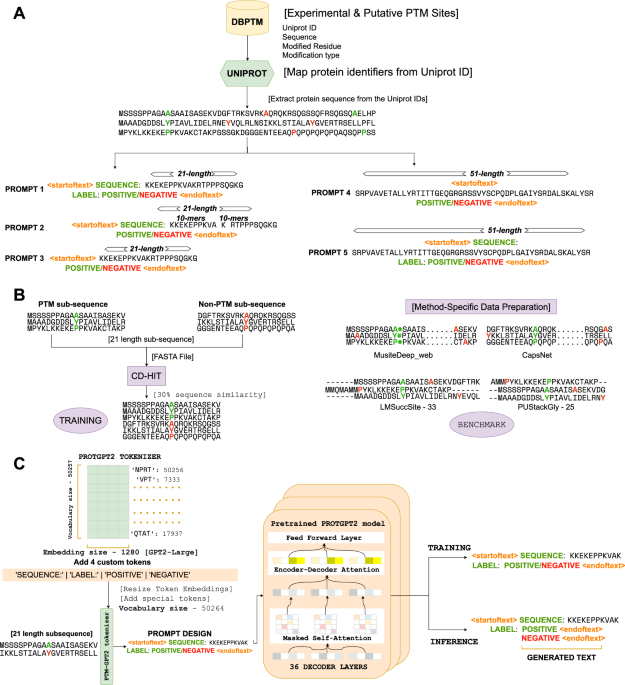
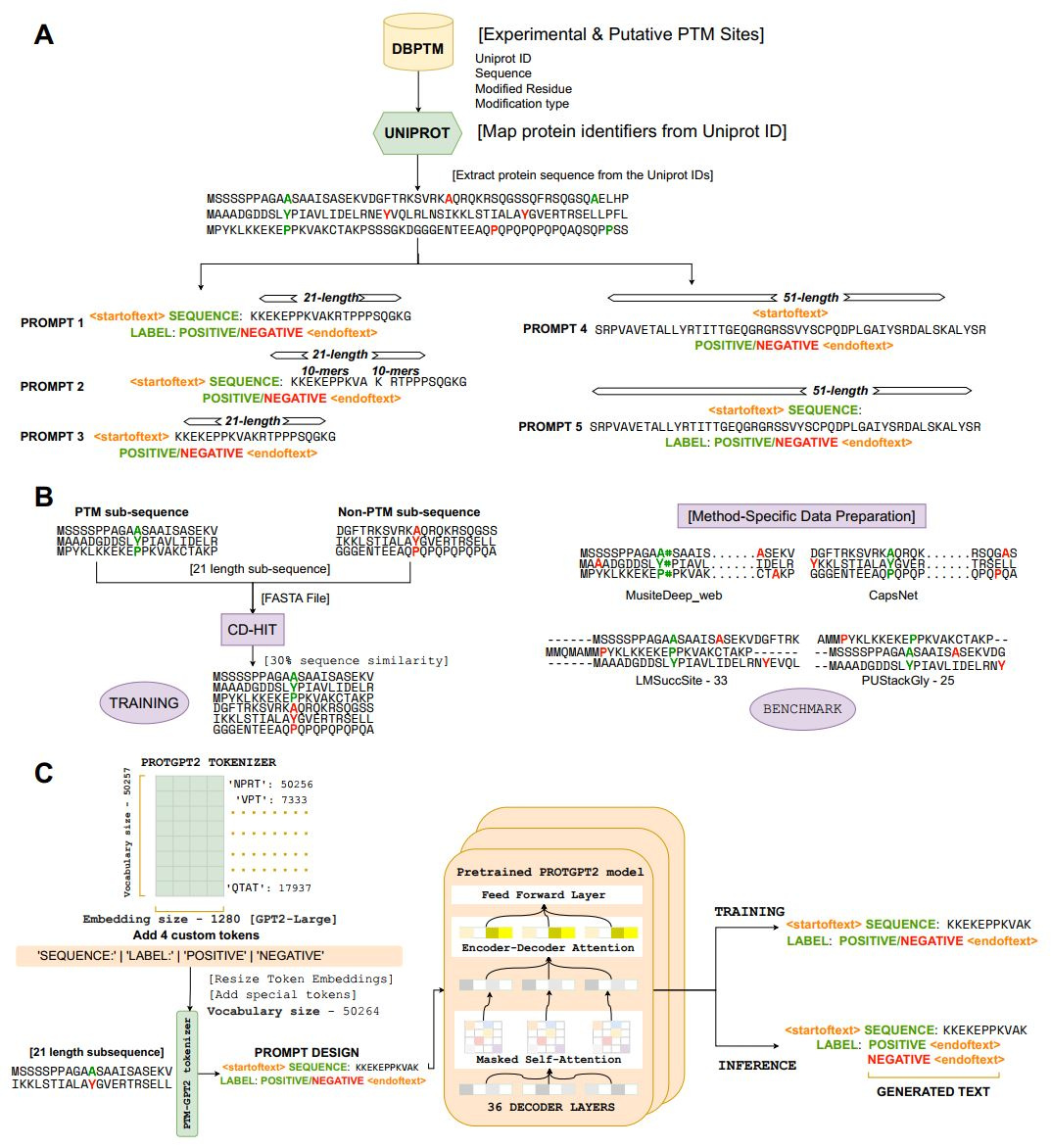
PTMGPT2 uses GPT-based architecture and prompt-based fine-tuning to predict post-translational modifications. It outperforms existing methods across 19 PTM types, offering interpretability and mutation analysis. This advances the understanding of protein function and disease research.
Published in Cell & Molecular Biology and Computational Sciences
Follow the Topic
Protein Biochemistry
Life Sciences > Biological Sciences > Molecular Biology > Protein Biochemistry
Artificial Intelligence
Mathematics and Computing > Computer Science > Artificial Intelligence
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
A selection of recent articles that highlight issues relevant to the treatment of neurological and psychiatric disorders in women.
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
This Collection aims to bring together research from various domains related to neurodegenerative conditions, encompassing novel insights into disease pathophysiology, diagnostics, therapeutic developments, and care strategies. We welcome the submission of all papers relevant to advances in neurodegenerative disease.
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in